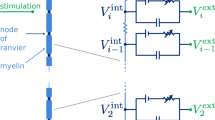
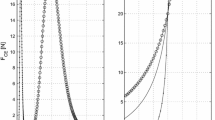
Abstract
In this study, the sensitivity of skeletal muscle fiber excitability to changes in temperature and a range of geometrical, electrical and ionic membrane properties was examined using model simulation. A mathematical model of the propagating muscle fiber action potential (AP) was used to simulate muscle fiber APs while changing individual muscle fiber parameters in isolation to examine how they affect muscle fiber AP amplitude, shape and conduction velocity (CV). The behavior of the model was verified by comparison with previously reported experimental data from both in vivo studies conducted at physiological temperatures and in vitro and in silico studies conducted at lower temperatures. The simulation results presented demonstrate the sensitivity of AP amplitude, shape and CV and, therefore, muscle fiber excitability to small changes in a wide range of different muscle fiber parameters. Furthermore, they demonstrate the potential of computational modeling as a tool for investigating the underlying mechanisms of complex phenomena such as those which govern skeletal muscle excitation.







Similar content being viewed by others
References
Adrian RH (1956) The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol 133:631–658
Adrian RH (1975) Conduction velocity and gating current in the squid giant axon. Proc R Soc Lond B Biol Sci 189:81–86
Adrian RH, Chandler WK, Hodgkin AL (1970) Slow changes in potassium permeability in skeletal muscle. J Physiol 208:645–668
Adrian RH, Peachey LD (1973) Reconstruction of the action potential of frog sartorius muscle. J Physiol 235:103–131
Arendt-Nielsen L, Gantchev N, Sinkjaer T (1992) The influence of muscle length on muscle fibre conduction velocity and development of muscle fatigue. Electroencephalogr Clin Neurophysiol 85:166–172
Arendt-Nielsen L, Mills KR (1988) Muscle fibre conduction velocity, mean power frequency, mean EMG voltage and force during submaximal fatiguing contractions of human quadriceps. Eur J Appl Physiol Occup Physiol 58:20–25
Barnes WS, Williams JH (1990) Staircase potentiation in isolated frog skeletal muscle: power spectral analysis of the evoked compound muscle action potential. Comp Biochem Physiol A Comp Physiol 96:387–394
Bigland-Ritchie B, Cafarelli E, Vollestad NK (1986) Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 556:137–148
Bigland-Ritchie B, Woods JJ (1984) Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7:691–699
Brandt NR, Caswell AH, Brunschwig JP (1980) ATP-energized Ca2+ pump in isolated transverse tubules of skeletal muscle. J Biol Chem 255:6290–6298
Bretag AH (1987) Muscle chloride channels. Physiol Rev 67:618–724
Brodie C, Sampson SR (1985) Contribution of electrogenic sodium–potassium ATPase to resting membrane potential of cultured rat skeletal myotubes. Brain Res 347:28–35
Cairns SP, Taberner AJ, Loiselle DS (2009) Changes of surface and t-tubular membrane excitability during fatigue with repeated tetani in isolated mouse fast- and slow-twitch muscle. J Appl Physiol 106:101–112
Dipolo R, Latorre R (1972) Effect of temperature on membrane potential and ionic fluxes in intact and dialysed barnacle muscle fibres. J Physiol 225:255–273
Dubois JM (1981) Simultaneous changes in the equilibrium potential and potassium conductance in voltage clamped Ranvier node in the frog. J Physiol 318:279–295
Edwards RH, Hill DK, Jones DA (1975) Heat production and chemical changes during isometric contractions of the human quadriceps muscle. J Physiol 251:303–315
Farina D, Arendt-Nielsen L, Graven-Nielsen T (2005) Effect of temperature on spike-triggered average torque and electrophysiological properties of low-threshold motor units. J Appl Physiol 99:197–203
Farina D, Falla D (2008) Effect of muscle-fiber velocity recovery function on motor unit action potential properties in voluntary contractions. Muscle Nerve 37:650–658
Fortune E, Lowery MM (2009) Effect of extracellular potassium accumulation on muscle fiber conduction velocity: a simulation study. Ann Biomed Eng 37:2105–2117
Gage PW, Eisenberg RS (1969) Action potentials, afterpotentials, and excitation–contraction coupling in frog sartorius fibers without transverse tubules. J Gen Physiol 53:298–310
Hakansson CH (1956) Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiol Scand 37:14–34
Hicks A, Fenton J, Garner S, McComas AJ (1989) M wave potentiation during and after muscle activity. J Appl Physiol 66:2606–2610
Hicks A, McComas AJ (1989) Increased sodium pump activity following repetitive stimulation of rat soleus muscles. J Physiol 414:337–349
Hille B (2001) Ion channels of excitable membranes, 3rd edn. Sinauer, Massachusetts
Hodgkin AL (1954) A note on conduction velocity. J Physiol 125:221–224
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol 108:37–77
Hodgkin AL, Nakajima S (1972) The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol 221:105–120
Huang CL, Peachey LD (1989) Anatomical distribution of voltage-dependent membrane capacitance in frog skeletal muscle fibers. J Gen Physiol 93:565–584
Juel C (1988) Muscle action potential propagation velocity changes during activity. Muscle Nerve 11:714–719
Juel C, Pilegaard H, Nielsen JJ, Bangsbo J (2000) Interstitial K(+) in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol 278:R400–R406
Jurkat-Rott K, Fauler M, Lehmann-Horn F (2006) Ion channels and ion transporters of the transverse tubular system of skeletal muscle. J Muscle Res Cell Motil 27:275–290
Kennedy PM, Cresswell AG (2001) The effect of muscle length on motor-unit recruitment during isometric plantar flexion in humans. Exp Brain Res 137:58–64
Knaflitz M, Merletti R, De Luca CJ (1990) Inference of motor unit recruitment order in voluntary and electrically elicited contractions. J Appl Physiol 68:1657–1667
Kossler F, Lange F, Caffier G, Kuchler G (1991) External potassium and action potential propagation in rat fast and slow twitch muscles. Gen Physiol Biophys 10:485–498
Kuntzer T, Flocard F, Vial C, Kohler A, Magistris M, Labarre-Vila A, Gonnaud PM, Ochsner F, Soichot P, Chan V, Monnier G (2000) Exercise test in muscle channelopathies and other muscle disorders. Muscle Nerve 23:1089–1094
Masuda K, Masuda T, Sadoyama T, Inaki M, Katsuta S (1999) Changes in surface EMG parameters during static and dynamic fatiguing contractions. J Electromyogr Kinesiol 9:39–46
McComas AJ, Galea V, Einhorn RW (1994) Pseudofacilitation: a misleading term. Muscle Nerve 17:599–607
McKenna MJ (1992) The roles of ionic processes in muscular fatigue during intense exercise. Sports Med 13:134–145
Merletti R, Knaflitz M, De Luca CJ (1990) Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol 69:1810–1820
Mortimer JT, Magnusson R, Petersen I (1970) Conduction velocity in ischemic muscle: effect on EMG frequency spectrum. Am J Physiol 219:1324–1329
Nandedkar SD, Stalberg E (1983) Simulation of single muscle fibre action potentials. Med Biol Eng Comput 21:158–165
Nielsen OB, Ortenblad N, Lamb GD, Stephenson DG (2004) Excitability of the T-tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+–K+ pump activity. J Physiol 557:133–146
Nishizono H, Kurata H, Miyashita M (1989) Muscle fiber conduction velocity related to stimulation rate. Electroencephalogr Clin Neurophysiol 72:529–534
Overgaard K, Nielsen OB, Flatman JA, Clausen T (1999) Relations between excitability and contractility in rat soleus muscle: role of the Na+–K+ pump and Na+/K+ gradients. J Physiol 518(Pt 1):215–225
Padmanabhan N, Huang CL (1990) Separation of tubular electrical activity in amphibian skeletal muscle through temperature change. Exp Physiol 75:721–724
Park EJ, Nishida T, Sufit RL, Minieka MM (2004) Prolonged compound muscle action potential duration in critical illness myopathy: report of nine cases. J Clin Neuromuscul Dis 5:176–183
Rutkove SB (2001) Effects of temperature on neuromuscular electrophysiology. Muscle Nerve 24:867–882
Rutkove SB, Kothari MJ, Shefner JM (1997) Nerve, muscle, and neuromuscular junction electrophysiology at high temperature. Muscle Nerve 20:431–436
Sakamoto K, Li W (1997) Effect of muscle length on distribution of muscle fiber conduction velocity for M. biceps brachii. Appl Human Sci 16:1–7
Schulte E, Farina D, Merletti R, Rau G, Disselhorst-Klug C (2004) Influence of muscle fibre shortening on estimates of conduction velocity and spectral frequencies from surface electromyographic signals. Med Biol Eng Comput 42:477–486
Sejersted OM, Sjogaard G (2000) Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 80:1411–1481
Stalberg E (1966) Propagation velocity in human muscle fibers in situ. Acta Physiol Scand Suppl 287:1–112
Stephanova DI, Dimitrov GV (1982) Mathematical modeling of ionic processes in human skeletal muscle fibres. Electromyogr Clin Neurophysiol 22:329–347
Wallinga W, Meijer SL, Alberink MJ, Vliek M, Wienk ED, Ypey DL (1999) Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys J 28:317–329
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fortune, E., Lowery, M.M. Effect of membrane properties on skeletal muscle fiber excitability: a sensitivity analysis. Med Biol Eng Comput 50, 617–629 (2012). https://doi.org/10.1007/s11517-012-0894-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-012-0894-8