Abstract
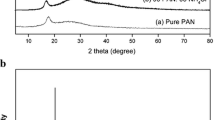
The conducting polymer electrolyte films consisting of polyacrylonitrile (PAN) as the host polymer, lithium triflate (LiCF3SO3) and sodium triflate (NaCF3SO3) as inorganic salts were prepared by the solution-cast technique. The pure PAN film was prepared as a reference. The ionic conductivity for the films is characterized using impedance spectroscopy. The room temperature conductivity for the PAN + 26 wt.% LiCF3SO3 film and the PAN + 24 wt.% NaCF3SO3 film is 3.04 × 10−4 S cm−1 and 7.13 × 10−4 S cm−1, respectively. XRD studies show that the complexation that has occurred in the PAN containing salt films and complexes formed are amorphous. The FTIR spectra results confirmed the complexation has taken place between the salt and the polymer. These results correspond with surface morphology images obtained from SEM analysis. The conductivity–temperature dependence of the highest conducting film from PAN + LiCF3SO3 and PAN + NaCF3SO3 systems follows Arrhenius equation in the temperature range of 303 to 353 K. The PAN containing 24 wt.% LiCF3SO3 film has a higher ionic conductivity and lower activation energy compared to the PAN containing 26 wt.%LiCF3SO3 film. These results can be explained based on the Lewis acidity of the alkali ions, i.e., the interaction between Li+ ion and the nitrogen atom of PAN is stronger than that of Na+ ion.





Similar content being viewed by others
References
Le Nest JP, Gandini A (1990) In: Scrosati B (ed) Proc. 2nd International Symposium on Polymer Electrolytes. Elsevier, Amsterdam, p 129
Scrosati B (1993) In: Scrosati B (ed) Applications of electroactive polymers. Chapman and Hall, London, p 251
Hooper A, Gauthier M, Belanger A (1990) In: Linford RG (ed) Electrochemical science and technology of polymers, vol 2. Elsevier, London, p 375
Murata K, Izuchi S, Yoshihisa Y (2000) Electrochim Acta 45:1501–1508
Wang C, Xia Y, Koumoto K, Sakai T (2002) J Electrochem Soc 149:A967–A972
Chen T, Chen-Yang YW (2004) Recent development of application of polymer electrolytes on polymer lithium secondary batteries. Huaxue 62(4):445–454
Hayamizu K, Aihara Y (2004) Electrochimica Acta 49:3397–3402
Fenton BE, Parker JM, Wright PW (1973) Polymer 14:589
Armand B (1979) In: Vashisthta P et al (eds) Fast ion transport in solids. Elsevier, North-Holland
Bandara LRAK, Dissayanake MAKL, Mellander BE (1998) Electrochimica Acta 43:1447–1451
Kumar B, Rodrigues SJ, Koka S (2002) Electrochimica Acta 47:4125–4131
Wang Y-J, Pan Y, Wang L, Pang M-J, Chen L (2005) Mater Lett 59:3021–3026
Vondrák J, Reiter J, Velicka J, Sedlaříkova M (2004) Solid State Ion 170:79–82
Osman Z, Md Isa KB, Ahmad A (2009) In: Arof AK, Majid SR, Teo LP, Kufian MZ (eds) Proc. of National Workshop on Functional Materials (NWFM). (ISBN 978-967-5148-43-9), pp 1-5
Abraham KM, Choe HS, Pasquariello DM (1998) Electrochim Acta 43:2399–2412
Chen-Yang YW, Chen HC, Lin FJ, Chen CC (2002) Solid State Ion 150:327–335
Yoon HK, Chung WS, Jo NJ (2004) Electrochim Acta 50:289–293
Huang B, Wang Z, Li G, Huang H, Xue R, Chen L, Wang F (1996) Solid State Ion 85:79–84
Ramya CS, Selvasekarapandian S, Savitha T, Hirankumar G (2007) Physica B 393:2672–2677
Souquet JL, Levy M, Duclot M (1994) Solid State Ion 70–71:337–345
Rajendran S, Sivakumar M, Subadevi R (2004) Mater Lett 58:641–649
Sagane F, Abe T, Iriyama Y, Ogumi Z (2005) J Power Sources 146:749–752
Abe T, Fukuda H, Iriyama Y, Ogumi Z (2004) J Electrochem Soc 151(8):A1120–A1123
Abe T, Ohtsuka M, Iriyama Y, Ogumi Z (2004) J Electrochem Soc 151(11):A1153–A1950
Berthier C, Gorecki W, Minier M, Armand MB, Chabagno JM, Rigaud P (1983) Solid State Ion 11:91–95
Rajendran S, Kannan R, Mahendran O (2001) Mater Lett 48:331–335
Acknowledgements
The authors would like to thank the University of Malaya and Academy of Sciences Malaysia for the grants and scholarship awarded.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osman, Z., Md Isa, K.B., Ahmad, A. et al. A comparative study of lithium and sodium salts in PAN-based ion conducting polymer electrolytes. Ionics 16, 431–435 (2010). https://doi.org/10.1007/s11581-009-0410-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0410-9