Abstract
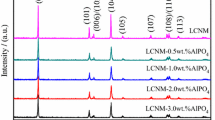
Al2O3 was successfully coated on LiNi0.8Co0.15Al0.05O2 cathode material by a heterogeneous nucleation-and-growth process with a core-shell structure for Li-ion battery. X-ray diffraction (XRD) measurements were used to indicate that the crystal structure of LiNi0.8Co0.15Al0.05O2 had no changes and no impurity phase existed after coating. Scanning electron microscopy (SEM) showed differences of surface morphology between coated and uncoated samples. A thin and bright coating layer was visually observed through transmission electron microscope (TEM). It reveals that the thickness of coating layer is 7 nm approximately. Electrochemical measurements were also carried out. Although the initial discharge capacity of the coated sample decreased, the 1-wt.% Al2O3-coated sample showed improved cycling performance at room temperature (25 °C) and elevated temperature (55 °C). It provided higher capacity retention of 71.7 and 70.1 % for 1C at 25 and 55 °C after 100 cycles, in comparison with 55.3 and 55.8 % for the uncoated sample. Meanwhile, interfacial resistance between active material and electrolyte decreased detected by electrochemical impedance spectroscopy (EIS) test. These enhancements in electrochemical characterizations are attributed to the improved stability of interface and the coating layer which served as the physical barriers to protect the active material from electrolyte attack.






Similar content being viewed by others
References
Bang HJ, Joachin H, Yang H, Amine K, Prakash J (2006) Contribution of the structural changes of LiNi0.8Co0.15Al0.05O2 cathodes on the exothermic reactions in Li-ion cells. J Electrochem Soc 153(4):A731–A737. doi:10.1149/1.2171828
Yoon WS, Chung KY, McBreen J, Yang XQ (2006) A comparative study on structural changes of LiCo1/3Ni1/3Mn1/3O2 and LiNi0.8Co0.15Al0.05O2 during first charge using in situ XRD. Electrochem Commun 8(8):1257–1262. doi:10.1016/j.elecom.2006.06.005
Kostecki R, McLarnon F (2004) Local-probe studies of degradation of composite LiNi0.8Co0.15Al0.05O2 cathodes in high-power lithium-ion cells. Electrochem Solid St 7(10):A380–A383. doi:10.1149/1.1793771
Zhuang GV, Chen GY, Shim J, Song XY, Ross PN, Richardson TJ (2004) Li2CO3 in LiNi0.8Co0.15Al0.05O2 cathodes and its effects on capacity and power. J Power Sources 134(2):293–297. doi:10.1016/j.jpowsour.2004.02.030
Zhang YC, Wang CY (2009) Cycle-life characterization of automotive lithium-ion batteries with LiNiO2 cathode. J Electrochem Soc 156(7):A527–A535. doi:10.1149/1.3126385
Myung ST, Cho MH, Hong HT, Kang TH, Kim CS (2005) Electrochemical evaluation of mixed oxide electrode for Li-ion secondary batteries: Li(1.1)Mn(1.9)O(4)and LiNi0.8Co0.15Al0.05O2. J Power Sources 146(1-2):222–225. doi:10.1016/j.jpowsour.2005.03.031
Ohzuku T, Ueda A, Nagayama M (1993) Electrochemistry and structural chemistry of Linio2 (R(3)over-Bar-M) for 4 volt secondary lithium cells. J Electrochem Soc 140(7):1862–1870. doi:10.1149/1.2220730
Itou Y, Ukyo Y (2005) Performance of LiNiCoO2 materials for advanced lithium-ion batteries. J Power Sources 146(1-2):39–44. doi:10.1016/j.jpowsour.2005.03.091
Andersson AM, Abraham DP, Haasch R, MacLaren S, Liu J, Amine K (2002) Surface characterization of electrodes from high power lithium-ion batteries. J Electrochem Soc 149(10):A1358–A1369. doi:10.1149/1.1505636
Arai H, Tsuda M, Saito K, Hayashi M, Sakurai Y (2002) Thermal reactions between delithiated lithium nickelate and electrolyte solutions. J Electrochem Soc 149(4):A401–A406. doi:10.1149/1.1452114
Aurbach D (2000) Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J Power Sources 89(2):206–218. doi:10.1016/S0378-7753(00)00431-6
Oh Y, Ahn D, Nam S, Park B (2010) The effect of Al2O3-coating coverage on the electrochemical properties in LiCoO2 thin films. J Solid State Electr 14(7):1235–1240. doi:10.1007/s10008-009-0946-7
Thackeray MM, Johnson CS, Kim JS, Lauzze KC, Vaughey JT, Dietz N, Abraham D, Hackney SA, Zeltner W, Anderson MA (2003) ZrO2- and Li2ZrO3-stabilized spinel and layered electrodes for lithium batteries. Electrochem Commun 5(9):752–758. doi:10.1016/S1388-2481(03)00179-6
Myung ST, Izumi K, Komaba S, Sun YK, Yashiro H, Kumagai N (2005) Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. Chem Mater 17(14):3695–3704. doi:10.1021/cm050566s
Lim SN, Ahn W, Yeon SH, Bin Park S (2014) Enhanced elevated-temperature performance of Li(Ni0.8Co0.15Al0.05)O-2 electrodes coated with Li2O-2B(2)O(3) glass. Electrochim Acta 136:1–9. doi:10.1016/j.electacta.2014.05.056
Cho Y, Cho J (2010) Significant improvement of LiNi0.8Co0.15Al0.05O2 cathodes at 60 degrees C by SiO2 dry coating for Li-ion batteries. J Electrochem Soc 157(6):A625–A629. doi:10.1149/1.3363852
Lee HJ, Nam SC, Park YJ (2011) Protection effect of ZrO2 coating layer on LiCoO2 thin film. B Korean Chem Soc 32(5):1483–1490. doi:10.5012/bkcs.2011.32.5.1483
Hu SK, Cheng GH, Cheng MY, Hwang BJ, Santhanam R (2009) Cycle life improvement of ZrO2-coated spherical LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries. J Power Sources 188(2):564–569. doi:10.1016/j.jpowsour.2008.11.113
Cho Y, Lee YS, Park SA, Lee Y, Cho J (2010) LiNi0.8Co0.15Al0.05O2 cathode materials prepared by TiO2 nanoparticle coatings on Ni0.8Co0.15Al0.05(OH)(2) Precursors. Electrochim Acta 56(1):333–339. doi:10.1016/j.electacta.2010.08.074
Xu Y, Li XH, Wang ZX, Guo HJ, Huang B (2015) Structure and electrochemical performance of TiO2-coated LiNi0.80CO0.15Al0.05O2 cathode material. Mater Lett 143:151–154. doi:10.1016/j.matlet.2014.12.093
Lee SH, Yoon CS, Amine K, Sun YK (2013) Improvement of long-term cycling performance of Li[Ni0.8Co0.15Al0.05]O-2 by AlF3 coating. J Power Sources 234:201–207. doi:10.1016/j.jpowsour.2013.01.045
Hu GR, Deng XR, Peng ZD, Du K (2008) Comparison of AlPO4- and Co-3(PO4)(2)-coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery. Electrochim Acta 53(5):2567–2573. doi:10.1016/j.electacta.2007.10.040
Wang JP, Du CY, Yan CQ, He XS, Song B, Yin GP, Zuo PJ, Cheng XQ (2015) Al2O3 coated concentration-gradient Li[Ni0.73Co0.12Mn0.15]O-2 cathode material by freeze drying for long-life lithium ion batteries. Electrochim Acta 174:1185–1191. doi:10.1016/j.electacta.2015.06.112
Qiu Q, Huang X, Chen YM, Tan Y, Lv WZ (2014) Al2O3 coated LiNi1/3Co1/3Mn1/3O2 cathode material by sol-gel method: preparation and characterization. Ceram Int 40(7):10511–10516. doi:10.1016/j.ceramint.2014.03.023
Tang YF, Huang ZP, Feng L, Chen YF (2005) Fabrication of alpha-AlO(OH)center dot SiO2 with core-shell structures by heterogeneous nucleation-and-growth processing. Appl Surf Sci 241(3-4):412–415. doi:10.1016/j.apsusc.2004.07.038
Tang YF, Li AD, Lu YN, Li XY, Shi SZ, Ling ZD (2003) Preparation of core/shell structure of alpha-Al(OH)(3)-SiO2 by heterogeneous nucleation-and-growth processing. J Sol-Gel Sci Techn 27(3):263–265. doi:10.1023/A:1024056617029
Tang YF, Lu YN, Li AD, Li XY, Shi SZ, Ling ZD (2002) Fabrication of fine mullite powders by alpha-Al(OH)(3)-SiO2 core-shell structure precursors. Appl Surf Sci 202(3-4):211–217. doi:10.1016/S0169-4332(02)00905-4
Tang YF, Li AD, Ling HQ, Wang YJ, Shao QY, Lu YN, Ling ZD (2002) Fabrication of composite particles with core-shell structures by a novel processing. J Mater Sci 37(16):3377–3379. doi:10.1023/A:1016597108481
Kim MH, Shin HS, Shin D, Sun YK (2006) Synthesis and electrochemical properties of Li[Ni0.8Co0.1Mn0.1]O-2 and Li[Ni0.8Co0.2]O-2 via co-precipitation. J Power Sources 159(2):1328–1333. doi:10.1016/j.jpowsour.2005.11.083
Yang SY, Wang XY, Yang XK, Bai YS, Liu ZL, Shu HB, Wei QL (2012) Determination of the chemical diffusion coefficient of lithium ions in spherical Li[Ni0.5Mn0.3Co0.2]O-2. Electrochim Acta 66:88–93. doi:10.1016/j.electacta.2012.01.061
Levi MD, Salitra G, Markovsky B, Teller H, Aurbach D, Heider U, Heider L (1999) Solid-state electrochemical kinetics of Li-ion intercalation into Li1-xCoO2: simultaneous application of electroanalytical techniques SSCV, PITT, and EIS. J Electrochem Soc 146(4):1279–1289. doi:10.1149/1.1391759
Aurbach D, Levi MD, Levi E, Teller H, Markovsky B, Salitra G, Heider U, Heider L (1998) Common electroanalytical behavior of Li intercalation processes into graphite and transition metal oxides. J Electrochem Soc 145(9):3024–3034. doi:10.1149/1.1838758
Zhuang QC, Wei T, Du LL, Cui YL, Fang L, Sun SG (2010) An electrochemical impedance spectroscopic study of the electronic and ionic transport properties of spinet LiMn2O4. J Phys Chem C 114(18):8614–8621. doi:10.1021/jp9109157
Zhao X, Zhuang QC, Wu C, Wu K, Xu JM, Zhang MY, Sun XL (2015) Impedance studies on the capacity fading mechanism of Li(Ni0.5Co0.2Mn0.3)O-2 cathode with high-voltage and high-temperature. J Electrochem Soc 162(14):A2770–A2779. doi:10.1149/2.0851514jes
Acknowledgments
This research was supported by the Jiangsu Province Prospective Joint Research on Pilot Project (No. BY2013072-03), a Grant for State Key Program for Basic Research of China (Nos. 2013CB632702 and 2012CB921503), the National Natural Science Foundation of China (No. 11134006), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), a project of free exploration funded by the National Laboratory of Solid State Microstructures, Test Foundation of Nanjing University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, G., Yu, M., Shen, F. et al. Improved cycling performance of LiNi0.8Co0.15Al0.05O2/Al2O3 with core-shell structure synthesized by a heterogeneous nucleation-and-growth process. Ionics 22, 2021–2026 (2016). https://doi.org/10.1007/s11581-016-1750-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1750-x