Summary
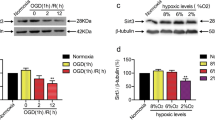
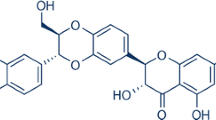
Salidroside is the active ingredient extracted from Rhodiola rosea, and has been reported to show protective effects in cerebral ischemia, but the exact mechanisms of neuronal protective effects are still unrevealed. In this study, the protective effects of salidroside (1 µmol/L) in ameliorating neuronal injuries induced by oxygen-glucose deprivation (OGD), which is a classical model of cerebral ischemia, were clarified. The results showed that after 8 h of OGD, the mouse hippocampal neuronal cell line HT22 cells showed increased cell death, accompanied with mitochondrial fragmentation and augmented mitophagy. However, the cell viability of HT22 cells showed significant restoration after salidroside treatment. Mitochondrial morphology and mitochondrial function were effectively preserved by salidroside treatment. The protective effects of salidroside were further related to the prevention of mitochondrial over-fission. The results showed that mTOR could be recruited to the mitochondria after salidroside treatment, which might be responsible for inhibiting excessive mitophagy caused by OGD. Thus, salidroside was shown to play a protective role in reducing neuronal death under OGD by safeguarding mitochondrial function, which may provide evidence for further translational studies of salidroside in ischemic diseases.
Similar content being viewed by others
References
Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y, et al.Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr Neuropharmacol, 2018,16(9):1396–1415
Deng F, Wang S, Zhang L, et al.Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: a literature review. J Cell Mol Med, 2017,21(9):1698–1710
Loscalzo J. Adaptions to Hypoxia and Redox Stress: Essential Concepts Confounded by Misleading Terminology. Circ Res, 2016,119(4):511–513
Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 2006,443(7113):787–795
Losón OC, Song Z, Chen H, et al.Newmeyer DD. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell, 2013,24(5):659–567
Yu R, Liu T, Jin SB, et al.MIEF1/2 function as adaptors to recruit Drp1 to mitochondria and regulate the association of Drp1 with Mff. Sci Rep, 2017,7(1):880
Li YJ, Lei YH, Yao N, et al.Autophagy and multidrug resistance in cancer. Chin J Cancer, 2017,36(1):52
Yoo SM, Jung YK. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol Cells, 2018,42(1):18–26
Um JH, Yun J. Emerging role of mitophagy in human diseases and physiology. BMB Rep, 2017,50(6):299–307
Guan R, Zou W, Dai X, et al.Mitophagy, a potential therapeutic target for stroke. J Biomed Sci, 2018,25(1):87
Naik PP, Birbrair A, Bhutia SK. Mitophagy-driven metabolic switch reprograms stem cell fate. Cell Mol Life Sci, 2019,76(1):27–43
Geisler S, Holmström KM, Skujat D, et al.PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol, 2010,12(2):119–131
Huang R, Xu Y, Wan W, et al.Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell, 2015,57(3):456–466
Huang W, Liao X, Tian J, et al.Traditional Chinese medicine for post-stroke depression: A systematic review and network meta-analysis (Protocol). Medicine (Baltimore), 2018,97(52):e13840
Xue H, Li P, Luo Y, et al.Salidroside stimulates the Sirt1/PGC-1alpha axis and ameliorates diabetic nephropathy in mice. Phytomedicine, 2019,54:240–247
Wei Y, Hong H, Zhang X, et al. Salidroside Inhibits Inflammation Through PI3K/Akt/HIF Signaling After Focal Cerebral Ischemia in Rats. Inflammation, 2017, 40(4):1297–1309
Zhong ZF, Han J, Zhang JZ, et al.Neuroprotective Effects of Salidroside on Cerebral Ischemia/Reperfusion-Induced Behavioral Impairment Involves the Dopaminergic System. Front Pharmacol, 2019,10:1433
Zheng T, Yang X, Wu D, et al.Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3beta pathway. Br J Pharmacol, 2015,172(13):3284–3301
Ju L, Wen X, Wang C, et al.Salidroside, A Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway. Front Pharmacol, 2017,8:749
Murakawa T, Yamaguchi O, Hashimoto A, et al.Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun, 2015,6:7527
Darko E, Heydarizadeh P, Schoefs B, et al.Photosynthesis under artificial light: the shift in primary and secondary metabolism. Philos Trans R Soc Lond B Biol Sci, 2014,369(1640):20130243
Ne N. Wu YZ, Jun Ren. Mitophagy, Mitochondrial Dynamics, and Homeostasis in Cardiovascular Aging. Oxid Med Cell Longev, 2019,2019:9825061
Roberts RF, Tang MY, Fon EA, et al.Defending the mitochondria: The pathways of mitophagy and mitochondrial-derived vesicles. Int J Biochem Cell Biol, 2016,79:427–436
Benjamin DSB, Fabian S, Tommy S, et al.Mitochondrial Oxidative Stress Impairs Energy Metabolism and Reduces Stress Resistance and Longevity of C. elegans. Oxid Med Cell Longev, 2019,2019:6840540
Rizwan H, Pal S, Sabnam S, et al.High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci, 2020,241:117148
Boya P, Esteban-Martínez L, Serrano-Puebla A, et al.Autophagy in the eye: Development, degeneration, and aging. Prog Retin Eye Res, 2016,55:206–245
Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci, 2015,16(6):345–357
Shen Z, Zheng Y, Wu J, et al.PARK2-dependent mitophagy induced by acidic postconditioning protects against focal cerebral ischemia and extends the reperfusion window. Autophagy, 2017,13(3):473–485
Wong KL, Akiyama R, Bessho Y, et al. ERK Activity Dynamics during Zebrafish Embryonic Development. Int J Mol Sci, 2018;20(1):109
Citraro R, Leo A, Constanti A, et al.mTOR pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis. Pharmacol Res, 2016,107:333–343
Cunningham JT, Rodgers JT, Arlow DH, et al.mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature, 2007,450(7170):736–740
Switon K, Kotulska K, Janusz-Kaminska A, et al.Molecular neurobiology of mTOR. Neuroscience, 2017,341:112–153
Ba L, Gao J, Chen Y, et al.Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine, 2019,58:152765
Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res, 2015,5(5):1602–1609
Head SA, Shi WQ, Yang EJ, et al.Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. ACS Chem Biol, 2016,12(1):174–182
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81873725, 81371416, and 31670778) and Hubei Province’s Outstanding Medical Academic Leader Program.
Conflict of Interest Statement
The authors declare no conflict of interest regarding the work.
Rights and permissions
About this article
Cite this article
Hu, Cy., Zhang, Qy., Chen, Jh. et al. Protective Effect of Salidroside on Mitochondrial Disturbances via Reducing Mitophagy and Preserving Mitochondrial Morphology in OGD-induced Neuronal Injury. CURR MED SCI 41, 936–943 (2021). https://doi.org/10.1007/s11596-021-2374-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2374-6