Abstract
Purpose
Normalization of the apparent diffusion coefficient (ADC) may overcome ADC variability attributable to different patient and/or technical factors. The purpose of this study was to compare the efficacy of ADC and the normalized ADC (nADC) for differentiating between prostate cancer with a Gleason score (GS) = 6 and GS > 6 and to identify an optimum reference for nADC calculations.
Materials and methods
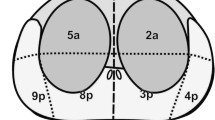
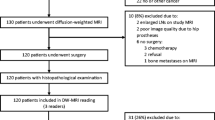
Our study population comprised 58 patients who underwent diffusion-weighted MRI followed by radical prostatectomy. The nADC of the prostate cancer was calculated as ADC (cancer)/ADC (reference) by using the obturator internus muscle, urine in the bladder, and a 20-ml saline bottle placed on the groin as references. We performed receiver operating characteristic (ROC) analysis to identify the optimum reference for nADC calculations.
Results
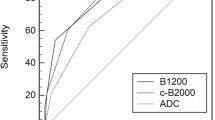
To differentiate between GS = 6 and GS > 6 prostate cancer, the area under the ROC curve of the nADC obtained with a saline bottle as reference was best (0.85) and significantly better than the area under the ADC ROC curve (0.71).
Conclusions
nADC is superior to ADC for estimating the aggressiveness of prostate cancer. It is a noninvasive technique that aids in the selection of appropriate treatments.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–57.
Itatani R, Namimoto T, Kajihara H, et al. Triage of low-risk prostate cancer patients with PSA levels 10 ng/ml or less: comparison of apparent diffusion coefficient value and transrectal ultrasound-guided target biopsy. AJR Am J Roentgenol. 2014;202(5):1051–7.
Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259(2):453–61.
Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258(2):488–95.
Kitajima K, Takahashi S, Ueno Y, et al. Clinical utility of apparent diffusion coefficient values obtained using high b-value when diagnosing prostate cancer using 3 tesla MRI: comparison between ultra-high b-value (2000 s/mm2) and standard high b-value (1000 s/mm2). J Magn Reson Imaging. 2012;36(1):198–205.
Hoehn-Berlage M, Eis M, Schmitz B. Regional and directional anisotropy of apparent diffusion coefficient in rat brain. NMR Biomed. 1999;12(1):45–50.
DeLano MC, Cooper TG, Siebert JE, et al. High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion coefficient map features. AJNR Am J Neuroradiol. 2000;21(10):1830–6.
Mulkern RV, Barnes AS, Haker SJ, et al. Biexponential characterization of prostate tissue water diffusion decay curves over an extended b-factor range. Magn Reson Imaging. 2006;24(5):563–8.
Thoeny HC, De Keyzer F, Oyen RH, et al. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology. 2005;235(3):911–7.
Bilgili Y, Unal B. Effect of region of interest on interobserver variance in apparent diffusion coefficient measures. AJNR Am J Neuroradiol. 2004;25(1):108–11.
Ulug AM, Beauchamp N Jr, Bryan RN, et al. Absolute quantitation of diffusion constants in human stroke. Stroke. 1997;28(3):483–90.
Tamada T, Sone T, Jo Y, et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging. 2008;28(3):720–6.
Oh J, Henry RG, Pirzkall A, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging. 2004;19(5):546–54.
Do RK, Chandarana H, Felker E, et al. Diagnosis of liver fibrosis and cirrhosis with diffusion-weighted imaging: value of normalized apparent diffusion coefficient using the spleen as reference organ. AJR Am J Roentgenol. 2010;195(3):671–6.
Barral M, Sebbag-Sfez D, Hoeffel C, et al. Characterization of focal pancreatic lesions using normalized apparent diffusion coefficient at 1.5-Tesla: preliminary experience. Diagn Interv Imaging. 2013;94(6):619–27.
Park SO, Kim JK, Kim KA, et al. Relative apparent diffusion coefficient: determination of reference site and validation of benefit for detecting metastatic lymph nodes in uterine cervical cancer. J Magn Reson Imaging. 2009;29(2):383–90.
Wang HJ, Pui MH, Guo Y, et al. Value of normalized apparent diffusion coefficient for estimating histological grade of vesical urothelial carcinoma. Clin Radiol. 2014;69(7):727–31.
Wang L, Mazaheri Y, Zhang J, et al. Assessment of biologic aggressiveness of prostate cancer: correlation of MR signal intensity with Gleason grade after radical prostatectomy. Radiology. 2008;246(1):168–76.
Jacobs MA, Ouwerkerk R, Petrowski K, et al. Diffusion-weighted imaging with apparent diffusion coefficient mapping and spectroscopy in prostate cancer. Top Magn Reson Imaging. 2008;19(6):261–72.
Haider MA, van der Kwast TH, Tanguay J, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189(2):323–8.
Kikuchi E, Scardino PT, Wheeler TM, et al. Is tumor volume an independent prognostic factor in clinically localized prostate cancer? J Urol. 2004;172(2):508–11.
Ohori M, Wheeler TM, Dunn JK, et al. The pathological features and prognosis of prostate cancer detectable with current diagnostic tests. J Urol. 1994;152(5 Pt 2):1714–20.
Hosseinzadeh K, Schwarz SD. Endorectal diffusion-weighted imaging in prostate cancer to differentiate malignant and benign peripheral zone tissue. J Magn Reson Imaging. 2004;20(4):654–61.
Zakian KL, Sircar K, Hricak H, et al. Correlation of proton MR spectroscopic imaging with gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology. 2005;234(3):804–14.
Braithwaite AC, Dale BM, Boll DT, et al. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology. 2009;250(2):459–65.
Sasaki M, Yamada K, Watanabe Y, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology. 2008;249(2):624–30.
Koh DM, Blackledge M, Collins DJ, et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol. 2009;19(11):2728–38.
Litjens GJ, Hambrock T, Hulsbergen-van de Kaa C, et al. Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology. 2012;265(1):260–6.
Kim CK, Park BK, Kim B. High-b-value diffusion-weighted imaging at 3T to detect prostate cancer: comparisons between b values of 1,000 and 2,000 s/mm2. AJR Am J Roentgenol. 2010;194(1):W33–7.
Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259(3):775–84.
Lim HK, Kim JK, Kim KA, et al. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection–a multireader study. Radiology. 2009;250(1):145–51.
Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255(1):89–99.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Itatani, R., Namimoto, T., Yoshimura, A. et al. Clinical utility of the normalized apparent diffusion coefficient for preoperative evaluation of the aggressiveness of prostate cancer. Jpn J Radiol 32, 685–691 (2014). https://doi.org/10.1007/s11604-014-0367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-014-0367-0