Abstract
Purpose
To evaluate whether quantitative analysis of perfusion contrast-enhanced ultrasound (CE-US) could predict early lymph-node (LN) metastasis in clinically node-negative breast cancer.
Materials and methods
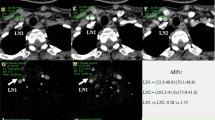
In this prospective study, 64 breast cancer patients were selected for perfusion CE-US imaging. Regions of interest were placed where the strongest and weakest signal increases were found to obtain peak intensities (PIs; PImax and PImin, respectively) for time–intensity curve analyzes. The PI difference and PI ratio were calculated as follows: PI difference = PImax−PImin; PI ratio = PImax/PImin.
Results
Forty-seven cases were histologically diagnosed as negative for LN metastasis and 17 were positive. There was a significant difference in PImin and the PI ratio between the LN-negative and -positive metastasis groups (p = 0.0053 and 0.0082, respectively). Receiver-operating curve analysis revealed that the area under the curve of PImin and the PI ratio were 0.73 and 0.72, respectively. The most effective threshold for the PI ratio was 1.52, and the sensitivity, specificity, positive predictive value, and negative predictive value were 59% (10/17), 87% (41/47), 63% (10/16), and 85% (41/48), respectively.
Conclusions
Parameters from the quantitative analysis of perfusion CE-US imaging showed significant differences between the LN-negative and -positive metastasis groups in clinically node-negative breast cancer.



Similar content being viewed by others
Abbreviations
- CE-US:
-
Contrast-enhanced ultrasound
- LN:
-
Lymph node
- PIs:
-
Peak intensities
- SLNB:
-
Sentinel LN biopsy
- TIC:
-
Time–intensity curve
- MVD:
-
Microvessel density
- AUC:
-
Area under the curve
- ROI:
-
Regions of interest
- ICC:
-
Interclass correlation coefficient
- ROC:
-
Receiver-operating characteristic
References
Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45(12):2917–24.
Banerjee M, George J, Song EY, et al. Tree-based model for breast cancer prognostication. J Clin Oncol. 2004;22:2567–75.
Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–1.
Swenson KK. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9:745–53.
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23.
Giuliano AE. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569.
Esen G, Gurses B, Yilmaz MH, et al. Gray scale and power Doppler US in the preoperative evaluation of axillary metastases in breast cancer patients with no palpable lymph nodes. Eur Radiol. 2005;15:1215–23.
Abe H, Schmidt RA, Kulkarni K, et al. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with us-guided 14-gauge core-needle biopsy—clinical experience in 100 patients 1. Radiology. 2009;250:41–9.
Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer. in vitro sonographic study. Am J Roentgenol. 2008;191:646–52.
Abe H, Schmidt RA, Sennett CA, et al. US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer: why and how to do it. Radiographics. 2007;27:S91–S99.
Scaranelo AM, Eiada R, Jacks LM, et al. Accuracy of unenhanced MR imaging in the detection of axillary lymph node metastasis: study of reproducibility and reliability. Radiology. 2012;262:425–34.
Shien T, Akashi-Tanaka S, Yoshida M, et al. Evaluation of axillary status in patients with breast cancer using thin-section CT. Int J Clin Oncol. 2008;13:314–9.
Loveless ME, Li X, Huamani J, et al. A method for assessing the microvasculature in a murine tumor model using contrast-enhanced ultrasonography. J Ultrasound Med. 2008;27:1699–709.
Szabó BK, Saracco A, Tánczos E, et al. Correlation of contrast-enhanced ultrasound kinetics with prognostic factors in invasive breast cancer. Eur Radiol. 2013;23:3228–36.
Li L, Mori S, Sakamoto M, et al. Mouse model of lymph node metastasis via afferent lymphatic vessels for development of imaging modalities. PLoS One. 2013;8:e55797.
Li L, Mori S, Kodama M, et al. Enhanced sonographic imaging to diagnose lymph node metastasis: importance of blood vessel volume and density. Cancer Res. 2013;73:2082–9.
Rubaltelli L, Beltrame V, Tregnaghi A, et al. Contrast-enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanoma. Am J Roentgenol. 2011;196:W8–W12.
Rubaltelli L, Corradin S, Dorigo A, et al. Automated quantitative evaluation of lymph node perfusion on contrast-enhanced sonography. Am J Roentgenol. 2007;188:977–83.
Wan CF, Du J, Fang H, et al. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012;262:450–9.
Pitre-Champagnat S, Leguerney I, Bosq J, et al. Dynamic contrast-enhanced ultrasound parametric maps to evaluate intratumoral vascularization. Investig Radiol. 2015;50:212–7.
Du J, Li F-H, Fang H, et al. Correlation of real-time gray scale contrast-enhanced ultrasonography with microvessel density and vascular endothelial growth factor expression for assessment of angiogenesis in breast lesions. J Ultrasound Med. 2008;27:821–3.
Mori N, Mugikura S, Takahashi S, et al. Quantitative analysis of contrast-enhanced ultrasound imaging in invasive breast cancer: a novel technique to obtain histopathologic information of microvessel density. Ultrasound Med Biol. 2017;43:607–14.
Ecanow JS, Abe H, Newstead GM, et al. Axillary staging of breast cancer: what the radiologist should know. Radiographics. 2013;33:1589–612.
Murphy CD, Jones JL, Javid SH, et al. Do sentinel node micrometastases predict recurrence risk in ductal carcinoma in situ and ductal carcinoma in situ with microinvasion? Am J Surg. 2008;196:566–8.
American College of Radiology. Breast imaging reporting and data system (BI-RADS). 5th ed. Reston: Reston American College of Radiology; 2013.
Schneider CA, Rasband WS, Eliceiri KW, et al. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159.
Ignee A, Jedrejczyk M, Schuessler G, et al. Quantitative contrast enhanced ultrasound of the liver for time intensity curves—reliability and potential sources of errors. Eur J Radiol. 2010;73:153–8.
Gadre A, Briner W, O’Leary M. A scanning electron microscope study of the human cervical lymph node. Acta Otolaryngol (Stockh.). 1994;114:87–90.
Herman PG, Kim C-S, de Sousa MA, et al. Microcirculation of the lymph node with metastases. Am J Pathol. 1976;85:333.
Ohta T, Nishioka M, Nakata N, et al. Five cases of axillary lymph node metastatic breast cancer on contrast-enhanced sonography. J Ultrasound Med. 2015;34:1131–7.
Goldberg BB, Merton DA, Liu J-B, et al. Contrast-enhanced sonographic imaging of lymphatic channels and sentinel lymph nodes. J Ultrasound Med. 2005;24:953–65.
Omoto K, Matsunaga H, Take N, et al. Sentinel node detection method using contrast-enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study. Ultrasound Med Biol. 2009;35:1249–56.
Cox K, Taylor-Phillips S, Sharma N, et al. Enhanced pre-operative axillary staging using intradermal microbubbles and contrast-enhanced ultrasound to detect and biopsy sentinel lymph nodes in breast cancer: a potential replacement for axillary surgery. Br J Radiol. 2018;91(1082):20170626.
Zhao J, Zhang J, Zhu Q-L, et al. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: a prospective study. Eur Radiol. 2018;28(4):1654–61.
Veronesi U, De Cicco C, Galimberti V, et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann Oncol. 2006;18:473–8.
Acknowledgements
This study was supported by JSPS KAKENHI 26461783 and 15K09913. The authors would like to thank Yumi Fujimoto in Tohoku University Hospital and Shomo Chou in Tohoku University for their kind support. We thank James P. Mahaffey, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Naoko Mori has nothing to disclose. Shunji Mugikura has nothing to disclose. Minoru Miyashita has nothing to disclose. Yumiko Kudo has nothing to disclose. Mikiko Suzuki has nothing to disclose. Li Li has nothing to disclose. Yu Mori has nothing to disclose. Shoki Takahashi has nothing to disclose. Kei Takase has nothing to disclose.
About this article
Cite this article
Mori, N., Mugikura, S., Miyashita, M. et al. Perfusion contrast-enhanced ultrasound to predict early lymph-node metastasis in breast cancer. Jpn J Radiol 37, 145–153 (2019). https://doi.org/10.1007/s11604-018-0792-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0792-6