Abstract
Background
Increasing availability of competing biosimilar alternatives makes it challenging to make treatment decisions. The purpose of this review is to evaluate the comparative efficacy and safety of ultra-long-/long-/intermediate-acting insulin products and biosimilar insulin compared to human/animal insulin in adults with type 1 diabetes mellitus (T1DM).
Methods
MEDLINE, EMBASE, CENTRAL, and grey literature were searched from inception to March 27, 2019. Randomized controlled trials (RCTs), quasi-experimental studies, and cohort studies of adults with T1DM receiving ultra-long-/long-/intermediate-acting insulin, compared to each other, as well as biosimilar insulin compared to human/animal insulin were eligible for inclusion. Two reviewers independently screened studies, abstracted data, and appraised risk-of-bias. Pairwise meta-analyses and network meta-analyses (NMA) were conducted. Summary effect measures were mean differences (MD) and odds ratios (OR).
Results
We included 65 unique studies examining 14,200 patients with T1DM. Both ultra-long-acting and long-acting insulin were superior to intermediate-acting insulin in reducing A1c, FPG, weight gain, and the incidence of major, serious, or nocturnal hypoglycemia. For fasting blood glucose, long-acting once a day (od) was superior to long-acting twice a day (bid) (MD - 0.44, 95% CI: - 0.81 to - 0.06) and ultra-long-acting od was superior to long-acting bid (MD - 0.73, 95% CI - 1.36 to - 0.11). For weight change, long-acting od was inferior to long-acting bid (MD 0.58, 95% CI: 0.05 to 1.10) and long-acting bid was superior to long-action biosimilar od (MD - 0.90, 95% CI: - 1.67 to - 0.12).
Conclusions
Our results can be used to tailor insulin treatment according to the desired results of patients and clinicians and inform strategies to establish a competitive clinical market, address systemic barriers, expand the pool of potential suppliers, and favor insulin price reduction.
PROSPERO Registration
CRD42017077051
Similar content being viewed by others
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is an autoimmune disease that gradually destroys beta cells in the pancreas leading to absolute insulin deficiency.1 Insulin replacement therapy mimics beta cell function by replacing both basal and prandial insulin secretion.2 Basal insulin replacement can be achieved with intermediate-acting insulin (e.g., isophane insulin Neutral Protamine Hagedorn; NPH, zinc insulin Lente),3 long-acting or ultra-long-acting insulin analogues (e.g., glargine, detemir,4 degludec).5 Regular human insulin, insulin analogues, and their biosimilar counterparts are complex biological molecules made from a similar manufacturing process.6 Structurally identical to their reference insulin analogues, biosimilar basal insulins were intended to function similarly to their reference insulin analogues6,7,8,9,10 and welcomed into the market for their potential cost-savings.11 However, biosimilar manufacturing has also been associated with differences in pharmacokinetic and pharmacodynamic properties, thereby potentially influencing insulin efficacy and safety,6,7,8,9,10 and the impact of this is unclear. We aimed to update our prior systematic review12 including biosimilars to evaluate the comparative efficacy and safety of ultra-long-/long-/intermediate-acting insulin compared to each other and biosimilar insulin.
METHODS
Protocol
Policy-makers from Health Canada and the Canadian Agency for Drugs and Technologies in Health (CADTH) commissioned the review, which was informed by the World Health Organization (WHO) insulin access initative.13
A protocol was prepared using the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P)14 and the Cochrane Handbook15 and registered with PROSPERO (CRD42017077051). Results are reported using the PRISMA-NMA16 and International Society for Pharmacoeconomics and Outcomes Research (ISPOR-NMA) tool.17
Eligibility Criteria
Patients
Adults (≥ 16 years of age) with T1DM for any duration of time, excluding animal studies.
Interventions
Ultra-long-/long-/intermediate-acting basal/bolus type of insulin therapy, with basal (taken between meals) and bolus (taken at mealtime) administered separately. Bolus insulin had to be specified and administered to all participants in all intervention and control groups. Bolus insulin included rapid- or short-acting insulin, while basal insulin included ultra-long, long- and intermediate-acting insulin. Insulin pumps or pre-mixed insulin preparations (e.g., long- and short-acting insulin combined) were excluded.
Comparator(s)/control
Ultra-long-/long-/intermediate-acting insulin, biosimilar insulin, no treatment.
Primary outcomes
Efficacy: glycemic control (glycated hemoglobin [A1c], FPG).
Secondary outcomes
Efficacy: all-cause mortality, diabetes-related morbidity (macrovascular, microvascular), health-related quality of life. Safety: weight change, hypoglycemia (all-cause, serious, minor, nocturnal), incident cancer, total adverse events (AEs), serious AEs, dropouts due to AEs.
Study designs
Experimental (randomized controlled trials [RCTs], non-randomized controlled trials, quasi-randomized trials), quasi-experimental (interrupted time series, controlled before and after studies), cohort studies.
Data Sources and Searches
Literature searches were developed by an experienced information scientist and peer-reviewed by a second using Peer Review of Electronic Search Strategies (PRESS)18 and executed in MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) (inception until March 27, 2019). Grey (i.e., difficult to locate/unpublished) literature was identified19 by searching the following: public health websites, drug regulatory websites, conference abstracts, and clinical trial registries. Reference lists of previous reviews and included studies were scanned. No restrictions on date, duration, or language were imposed.
Study Selection
Pairs of reviewers independently screened literature search results using Synthesi.SR software.20 A calibration exercise was conducted on 50 titles/abstracts and 80% agreement was achieved. Two team members independently screened remaining citations with 90% agreement. Two calibration exercises were completed using 20 eligible full-text articles each, until 65% agreement was achieved. Remaining full-text articles were screened independently by two reviewers with 81% agreement. Conflicts were resolved through discussion or by a third reviewer.
Quality Assessment
Risk-of-bias (ROB) of included studies was appraised independently by two reviewers using the Cochrane ROB tool for RCTs, Cochrane Effective Practice and Organization of Care (EPOC) tool for non-randomized controlled trials, and the Newcastle-Ottawa Scale for cohort studies. Conflicts were resolved by discussion or by a third reviewer.
Data Items and Abstraction
Data were abstracted on study characteristics, patient characteristics, and outcome results (e.g., A1c) including their definitions for the longest duration of follow-up.15 A draft data abstraction form was created and a calibration exercise was completed for five studies. Subsequently, two reviewers independently abstracted relevant outcome data for each included study. A third reviewer resolved conflicts. Authors were emailed for missing data or data clarifications.
Data Analysis
Pairwise Meta-Analysis
Due to the small number of studies available for meta-analyses (MAs),21 fixed-effect MAs were conducted for direct pairwise comparisons of treatments using the odds ratio (OR) effect measure for dichotomous data and the mean difference (MD) for continuous data,15 and adjusted for the effect of the bolus covariate (rapid versus short) using meta-regression.15
For a cross-over RCT to contribute outcome data, the trial had to account for the paired nature (i.e., repeated, subject-level measurements) of the study design in conducting and reporting the study-specific effect estimate and its corresponding measure of uncertainty (e.g., confidence interval [CI], standard error),22 which was verified by a statistician (ZB). For studies with dichotomous outcomes that reported zero events in one treatment arm, Stata23 automatically added 0.5 to the numerator and one to the denominator. Studies reporting that participants in both treatment arms experienced 0% events or 100% events were excluded from the analysis.15 Heterogeneity was assessed using the I2 statistic.24
NMA
For a connected network with the number of studies exceeding the treatment nodes, a network diagram was drawn and random-effects network meta-analysis (NMA) was performed.25, 26 Treatment nodes27, 28 were selected by clinicians to capture the major insulin class, origin of the insulin, and administration frequency. Two sets of analyses were conducted; one based on basal insulin class and the other based on insulin class/origin/frequency; Table 1. A common within-network estimate for the heterogeneity parameter across comparisons was estimated with the restricted maximum likelihood (REML) method. Transitivity assumption was assessed by examining distributions of treatment effect modifiers across comparisons.25, 29,30,31 Global consistency was assessed between direct and indirect evidence across the entire network using the design-by-treatment interaction model.32, 33 Statistically significant global inconsistency led to local consistency being assessed via the loop-specific method.34 Treatment effect estimates, 95% CIs, and 95% predictive intervals (PrI) were calculated.16 To assess the presence of small-study effects, a comparison-adjusted funnel plot was drawn per outcome.29, 35 Within each plot, comparison-adjusted treatment effect estimates were ordered from earliest to most recent according to Health Canada/Federal Drug Administration approval dates. Analyses were performed using Stata version 15.1 using the following packages: metan, metareg, mvmeta, and network commands.23, 35, 36 Additional analysis methods are available in Appendix Methods 1.
Role of the Funding Source
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit for publication.
RESULTS
Literature Search
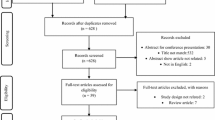
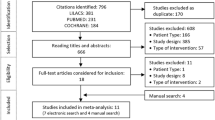
The literature search identified 21,346 titles/abstracts, of which 1170 were potentially relevant (Fig. 1). Sixty-five unique studies and 13 companion reports were included (Appendix File 1); 27 and 37 studies were included in the basal insulin class and insulin class/origin/frequency analyses, respectively. Six37,38,39,40,41,42 non-English studies and two protocols43, 44 were included. Authors of 89 studies were emailed and responses were received for 18 studies; one45 provided additional data for the analysis.
Patient Characteristics
Across the included studies, sample sizes ranged from eight to 749, with a total of 14,200 patients. The proportion of females ranged from 0 to 100%, average age ranged from 23 to 54 years, average baseline A1c ranged from 7 to 10%, average body mass index (BMI) ranged from 22 to 28, and duration of T1DM ranged from 8 to 27 years (Table 2, Appendix Table 1).
Study Characteristics
Publication years of the 65 included studies ranged from 1984 to 2018. Sixty-four studies were RCTs, of which 41 (64%) were parallel RCTs and 23 (36%) were cross-over RCTs. One study was a non-randomized controlled trial. Most of the studies took place across multiple centers in Europe and North America, with few studies from low-and-middle-income countries (LMICs) (Table 2, Appendix Table 2).
ROB and Quality Appraisal Results
A score of unclear/high ROB was given for the majority of RCTs regarding allocation concealment (75%), blinding of participants and personnel (78%), blinding of outcome assessment (44%), incomplete outcome data (28%), selective reporting (63%), and “other” bias (e.g., funding bias, 92%) (Appendix Table 3, Appendix Figure 1). A single non-randomized controlled trial was assessed using the Cochrane EPOC ROB Tool, which scored unclear for 7/9 items and high ROB for random sequence generation and incomplete outcome data (Appendix Table 4).
NMA Results
All statistically significant results of NMA for each outcome are provided (Tables 3 and 4). In Table 3, a summary of all treatment comparisons and outcomes is provided and whether the results were statistically significant or not. Unless otherwise noted, the type of insulin was human. All results whether statistically significant or not can be found in Appendix Data 1, Appendix Tables 5–17, and Appendix Figures 2–9.
Primary Outcomes
A1c
For basal insulin class, NMA for the A1c outcome included 25 RCTs and 8327 patients. Long-acting insulin had a greater A1c reduction compared to intermediate-acting insulin (MD - 0.14, 95% CI: - 0.22 to - 0.06) (Appendix Tables 5–8, Appendix Figures 3–4). In addition, ultra-long-acting insulin had a greater A1c reduction compared to intermediate-acting insulin (MD - 0.08, 95% CI: - 0.25 to 0.10) but not long-acting insulin (MD 0.06, 95% CI: - 0.10 to 0.22).
For a specific type of insulin, NMA was conducted on the A1c outcome and included 34 RCTs and 11,654 patients and 9 treatment nodes (Appendix Table 14, Appendix Figure 7). Transitivity assumption was verified and there was no evidence of inconsistency (Appendix Table 13, Appendix Figure 6), yet there might be bias associated with small-study effects (Appendix Figure 8). There were 36 treatment comparisons and the following demonstrated a statistically significant difference:
Intermediate-acting (human) once a day (od) was inferior to:
-
1.
Intermediate-acting (human) twice a day, bid (MD 0.11, 95% CI: 0.01 to 0.21)
Intermediate-acting (human) four-times a day (qid) was inferior to:
-
2.
Intermediate-acting (animal and human), bid (MD 0.31, 95% CI: 0.06 to 0.56)
-
3.
Intermediate-acting (human), od (MD 0.32, 95% CI: 0.12 to 0.53)
-
4.
Intermediate-acting (human), bid (MD 0.43, 95% CI: 0.24 to 0.63)
Long-acting (human) od was superior to:
-
5.
Intermediate-acting (animal and human), bid (MD - 0.19, 95% CI - 0.36 to - 0.02)
-
6.
Intermediate-acting (human), od (MD - 0.18, 95% CI - 0.27 to - 0.08)
-
7.
Intermediate-acting (human), qid (MD - 0.50, 95% CI - 0.68 to - 0.32)
Long-acting (human) bid was superior to:
-
8.
Intermediate-acting (animal), bid (MD - 1.27, 95% CI - 2.53 to - 0.01)
-
9.
Intermediate-acting (animal and human), bid (MD - 0.19, 95% CI - 0.37 to - 0.01)
-
10.
Intermediate-acting (human), od (MD - 0.18, 95% CI - 0.29 to - 0.07)
-
11.
Intermediate-acting (human), bid (MD - 0.07, 95% CI - 0.13 to - 0.01)
-
12.
Intermediate-acting (human), qid (MD - 0.50, 95% CI - 0.70 to - 0.30)
Long-acting (biosimilar) od was superior to:
-
13.
Intermediate-acting (human), od (MD - 0.14, 95% CI - 0.27 to - 0.01)
-
14.
Intermediate-acting (human), qid (MD - 0.46, 95% CI - 0.66 to - 0.26)
-
15.
Ultra-long-acting (human) od was superior to:
-
16.
Intermediate-acting (human), od (MD - 0.14, 95% CI - 0.28 to - 0.01)
-
17.
Intermediate-acting (human), qid (MD - 0.47, 95% CI - 0.67 to - 0.26)
Several sensitivity analyses (Appendix Table 15) were conducted to examine the impact of imputing missing standard deviations (SDs) on the results, controlling for studies involving Lente insulin or cross-over RCTs. This resulted in the exclusion of 7 to 8 trials for each sensitivity analysis. The direction of the effect was maintained; however, all statistically significant pairwise treatment comparisons reported above were no longer statistically significant, likely because of the small number of remaining trials. Sub-group analyses (Appendix Table 16) were conducted for bolus type, follow-up duration, study design, ROB associated with random sequence generation and allocation concealment, age, proportion of women, duration of diabetes, and A1c level (mild: <8%, severe: ≥8%); none of the results was statistically significant.
Fasting Plasma Glucose (FPG)
For basal insulin class, NMA for the FPG outcome included 21 RCTs, 7685 patients, and three treatment nodes. Long-acting insulin had a greater FPG reduction compared to intermediate-acting insulin (MD - 1.03, 95% CI: - 1.33 to - 0.73) and ultra-long-acting insulin had a greater FPG reduction compared to intermediate-acting insulin (MD - 1.45, 95% CI: - 2.12 to - 0.79) and long-acting insulin (MD - 0.42, 95% CI: - 1.02 to 0.18) (Appendix Tables 6–7, Appendix Figures 3–4).
For a specific type of insulin, NMA was conducted on the FPG outcome and included 29 RCTs, 10,290 patients, and 8 treatment nodes (Appendix Table 14, Appendix Figure 7). Transitivity assumption was verified and there was no evidence of inconsistency (Appendix Table 13, Appendix Figure 6), yet there might be bias associated with small-study effects (Appendix Figure 8). There were 28 treatment comparisons and the following demonstrated a statistically significant difference:
Long-acting (human) od was superior to:
-
1.
Intermediate-acting (human), od (MD - 1.15, 95% CI: - 1.79 to - 0.50)
-
2.
Intermediate-acting (human), bid (MD - 1.26, 95% CI: - 1.65 to - 0.87)
-
3.
Intermediate-acting (human), qid (MD - 0.90, 95% CI: - 1.79 to - 0.01)
-
4.
Long-acting (human), bid (MD - 0.44, 95% CI: - 0.81 to - 0.06)
Long-acting (human) bid was superior to:
-
5.
Intermediate-acting (human), bid (MD - 0.82, 95% CI: - 1.20 to - 0.44)
Long-acting (biosimilar) od was superior to:
-
6.
Intermediate-acting (human), od (MD - 1.05, 95% CI: - 1.95 to - 0.16)
-
7.
Intermediate-acting (human), bid (MD - 1.16, 95% CI: - 1.91 to - 0.42)
Ultra-long-acting (human) od was superior to:
-
8.
Intermediate-acting (human), od (MD - 1.44, 95% CI: - 2.31 to - 0.58)
-
9.
Intermediate-acting (human), bid (MD - 1.55, 95% CI: - 2.22 to - 0.89)
-
10.
Intermediate-acting (human), qid (MD - 1.20, 95% CI: - 2.26 to - 0.13)
-
11.
Long-acting (human), bid (MD - 0.73, 95% CI - 1.36 to - 0.11)
Secondary Outcomes
Weight Change
For basal insulin class, NMA was conducted on weight change with 15 RCTs, 5908 patients, and three treatment nodes. Long-acting insulin reduced weight gain compared to intermediate-acting insulin (MD - 0.70, 95% CI: - 1.08 to - 0.32) (Appendix Table 6, Appendix Figure 3). Ultra-long-acting insulin reduced weight gain compared to intermediate-acting insulin (MD - 0.53, 95% CI: - 1.25 to 0.18) but not long-acting insulin (MD 0.17, 95% CI: - 0.44 to 0.77). Four studies were removed in sensitivity analysis due to the potential for bias associated with small-study effects; ultra-long-acting insulin was statistically superior to intermediate-acting insulin (MD - 0.80, 95% CI: - 1.29 to - 0.32) (Appendix Table 7, Appendix Figure 4).
For a specific type of insulin, NMA was conducted on weight change with 20 RCTs, 7938 patients, and 7 treatment nodes (Appendix Table 14, Appendix Figure 7). Transitivity assumption was verified and there was no evidence of inconsistency (Appendix Table 13, Appendix Figure 6), yet there might be bias associated with small-study effects (Appendix Figure 8). There were 21 treatment comparisons and the following demonstrated a statistically significant difference:
Long-acting (human) od was inferior to:
-
1.
Long-acting (human), bid (MD 0.58, 95% CI: 0.05 to 1.10)
Long-acting (human) bid was superior to:
-
2.
Intermediate-acting (human), od (MD - 1.22, 95% CI: - 2.11 to - 0.32)
-
3.
Intermediate-acting (human), bid (MD - 0.86, 95% CI: - 1.23 to - 0.48)
-
4.
Long-acting (biosimilar), od (MD - 0.90, 95% CI: - 1.67 to - 0.12)
Major or Serious Hypoglycemia
For basal insulin class, NMA was conducted on the major or serious hypoglycemia outcome (defined in Appendix Table 12) with 16 RCTs, 6900 patients, and three treatment nodes. Long-acting insulin was associated with a reduced incidence of major or serious hypoglycemic episodes compared to intermediate-acting insulin (OR 0.63, 95% CI: 0.51 to 0.79) (Appendix Tables 6–7, Appendix Figures 3–4). Ultra-long-acting insulin reduced major or serious hypoglycemic episodes compared to intermediate-acting insulin (OR 0.71, 95% CI: 0.43 to 1.17) but not compared to long-acting insulin (OR 1.12, 95% CI: 0.71 to 1.77).
For a specific type of insulin, NMA was conducted on major or serious hypoglycemia with 20 RCTs, 8240 patients, and 6 treatment nodes (Appendix Table 14, Appendix Figure 7). Transitivity assumption was verified and there was no evidence of inconsistency (Appendix Table 13, Appendix Figure 6), yet there might be bias associated with small-study effects (Appendix Figure 8). There were 15 treatment comparisons and the following demonstrated a statistically significant difference:
Long-acting (human) od was associated with less incidence when compared to:
-
1.
Intermediate-acting (human), od (OR 0.61, 95% CI: 0.40 to 0.94)
-
2.
Intermediate-acting (human), bid (OR 0.56, 95% CI: 0.39 to 0.80)
Long-acting (human) bid was associated with less incidence when compared to:
-
3.
Intermediate-acting (human), bid (OR 0.70, 95% CI: 0.52 to 0.93)
Nocturnal Hypoglycemia
For basal insulin class, NMA was conducted for nocturnal hypoglycemia (defined in Appendix Table 12) with 13 RCTs, 5423 patients, and three treatment nodes. Long-acting insulin (OR 0.74, 95% CI: 0.58 to 0.94) and ultra-long-acting insulin (OR 0.64, 95% CI: 0.41 to 0.99) lowered the incidence of nocturnal hypoglycemic episodes compared to intermediate-acting insulin (Appendix Tables 6–7, Appendix Figures 3–4). In addition, ultra-long-acting insulin was associated with a lower risk of nocturnal hypoglycemic episodes compared to long-acting insulin (OR 0.86, 95% CI: 0.60 to 1.24).
For a specific type of insulin, NMA was conducted on nocturnal hypoglycemia with 16 RCTs, 6318 patients, and 6 treatment nodes (Appendix Table 14, Appendix Figure 7). Transitivity assumption was verified and there was no evidence of inconsistency (Appendix Table 13, Appendix Figure 6). However, there might be bias associated with small-study effects (Appendix Figure 8). Across 15 treatment comparisons, only one was statistically significant and long-acting administered bid was associated with less incidence when compared to intermediate-acting administered bid (OR 0.61, 95% CI: 0.43 to 0.87).
Other Secondary Outcomes
No statistically significant results were found across treatment comparisons where NMA and or MA was done for the following outcomes: mortality, any vascular complications, microvascular complications, macrovascular complications, quality-of-life, all-cause hypoglycemia, minor or mild hypoglycemia, incident cancers, any AEs, serious AEs, and dropout due to AEs.
Rank-Heat Plots
Across basal insulin class NMAs, the results suggest that long-acting insulin has the greatest likelihood of being the most effective and safest (Appendix Figure 5). Across the insulin class/origin/frequency NMAs, the results suggested that long-acting biosimilar insulin administered once daily had the greatest likelihood of being the most effective and safe (Appendix Figure 9).
DISCUSSION
For basal insulin classes, long-acting insulin was superior to intermediate-acting insulin across the outcomes including A1c, FPG, weight, major or serious hypoglycemia, and nocturnal hypoglycemia. In addition, ultra-long-acting insulin was statistically superior to intermediate-acting insulin for FPG and nocturnal hypoglycemia. For fasting blood glucose, long-acting od was superior to long-acting bid and ultra-long-acting od was superior to long-acting bid. For weight change, long-acting od was inferior to long-acting bid and long-acting bid was superior to long-action biosimilar od. These results are inconsistent with recent clinical practice guidelines that recommend ultra-long-acting insulin,2 likely because the guidelines included patients with T1DM and type 2 diabetes.46 We included the same two studies looking at T1DM,47, 48 as well as an additional 10 studies.49,50,51,52,53,54,55,56,57,58 The rank-heat plots suggest that long-acting insulin had the greatest likelihood of being the most effective and safest compared with intermediate-acting insulin and ultra-long-acting insulin. For the specific types of insulin, long-acting biosimilar od had the greatest likelihood of being the most effective and safest according to the rank-heat plot. Only one statistically significant difference was observed between the biosimilar insulin and human/animal insulin, which was for weight change, and is inconsistent with previous research on biosimilars.7
In LMICs, food insecurity might hamper the regular alternation of food needed when NPH is used to provide the basal concentration of insulin. NPH is associated with more severe hypoglycemia events. Furthermore, access to glucagon, a high-cost product, in case of severe hypoglycemia, is limited in many low-resource settings.59 Long-acting analogues of insulin, even when administered od, allow for more flexibility in the number and timing of meals and can be associated with better compliance when compared to NPH. The WHO set a target to reduce the risk of premature noncommunicable disease death by 25% by 2025.60 To achieve this, the focus must now be on the implementation strategies of insulins (and other interventions), and reducing uncertain access related to affordability and prices. Various mechanisms like bulk purchasing contracts, biosimilar availability, increasing tender competition and identifying reasonable rebates can protect financial stability for both patients and countries.
Our systematic review has several strengths, including following the Cochrane Handbook15 and ISPOR guidance.17 However, our results should be interpreted with caution, as many of the NMAs had a small number of included studies and many had high ROB on several items. Other limitations include that we were unable to abstract data for cardiovascular-related mortality and healthcare utilization and that our results may have been impacted by the inclusion of a large number of cross-over studies, animal bolus insulin studies, and assumption that the bolus insulin was human if it was not specified in the study.
In conclusion, ultra-long-acting and long-acting insulin were superior to intermediate-acting insulin. Furthermore, long-acting od is more effective than long-acting bid and ultra-long-acting od is more effective than long-acting bid for fasting blood glucose. For weight change, long-acting od was less effective than long-acting bid and long-acting bid was more effective than long-action biosimilar od. Our results can be used by patients and healthcare providers to tailor their choice of insulin treatment according to a desired outcome. To attain the WHO goal, policy-makers must activate policies supporting access to insulins by making them accessible and affordable.
Abbreviations
- A1c:
-
glycated hemoglobin
- AE:
-
adverse event
- CADTH:
-
Canadian Agency for Drugs and Technologies in Health
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CI:
-
confidence interval
- EPOC:
-
Cochrane Effective Practice and Organization of Care tool
- FPG:
-
fasting plasma glucose
- ISPOR:
-
International Society for Pharmacoeconomics and Outcomes Research
- MD:
-
mean difference
- MA:
-
meta-analysis
- NPH:
-
Neutral Protamine Hagedorn
- NMA:
-
network meta-analysis
- OR:
-
odds ratio
- PrI:
-
predictive interval
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-analysis
- RCT:
-
randomized controlled trial
- REML:
-
restricted maximum likelihood
- ROB:
-
risk of bias
- SUCRA:
-
surface under the cumulative ranking curves
- T1DM:
-
type 1 diabetes mellitus
- WHO:
-
World Health Organization
References
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S13-S27.
Angela McGibbon M, Lenley Adams M, Karen Ingersoll R, Barna Tugwell M. Glycemic management in adults with type 1 diabetes. Can J Diabetes 2018;42:S80-S7.
Sanches AC, Correr CJ, Venson R, Pontarolo R. Revisiting the efficacy of long-acting insulin analogues on adults with type 1 diabetes using mixed-treatment comparisons. Diabetes Res Clin Pract 2011;94(3):333-9.
Vardi M, Jacobson E, Nini A, Bitterman H. Intermediate acting versus long acting insulin for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2008(3):Cd006297.
Bode BW, Buse JB, Fisher M, et al. Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal-bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN((R)) Basal-Bolus Type 1): 2-year results of a randomized clinical trial. Diabet Med 2013;30(11):1293-7.
James J, Pollom RK, Hadjiyianni I, Buchholz G, Reed BL. Biosimilar insulins: What do you need to know? Int Diabetes Nursing 2017;14(1):32-5.
Tieu C, Lucas EJ, DePaola M, Rosman L, Alexander GC. Efficacy and safety of biosimilar insulins compared to their reference products: A systematic review. PLoS One 2018;13(4):e0195012.
Yamada T, Kamata R, Ishinohachi K, et al. Biosimilar vs originator insulins: Systematic review and meta-analysis. Diabetes Obes Metab 2018;20(7):1787-92.
Dolinar R, Lavernia F, Edelman S. A Guide to follow-on biologics and biosimilars with a focus on insulin. Endocr Pract 2018;24(2):195-204.
Heinemann L, Hompesch M. Biosimilar Insulins: Basic Considerations. J Diabetes Sci Technol 2014;8(1):6-13.
White J, Goldman J. Biosimilar and follow-on insulin: The ins, outs, and interchangeability. J Pharm Technol 2019;35(1):25-35.
Tricco AC, Ashoor HM, Antony J, et al. Safety, effectiveness, and cost effectiveness of long acting versus intermediate acting insulin for patients with type 1 diabetes: systematic review and network meta-analysis. BMJ. 2014;349:g5459.
Word Health Organization. Pilot Procedure for Prequalification of Human Insulin. 2020. [Available from: https://www.who.int/medicines/regulation/prequalification/pq_human_insulin/en/].
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from https://www.handbook.cochrane.org.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162(11):777-84.
Jansen JP, Trikalinos T, Cappelleri JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014;17(2):157-73.
Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol 2009;62(9):944-52.
Grey matters: a practical tool for searching health-related grey literature. Ottawa: CADTH; 2018. [Available from: https://www.cadth.ca/resources/finding-evidence].
Synthesi.SR. Toronto, Canada: Knowledge Translation Program, St. Michael's Hospital; 2012.
Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res Synth Methods 2017;8(3):290-302.
Li T, Yu T, Hawkins BS, Dickersin K. Design, Analysis, and Reporting of Crossover Trials for Inclusion in a Meta-Analysis. PLoS One 2015;10(8):e0133023.
StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(11):1539-58.
Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence Synthesis for Decision Making 2:A Generalized Linear Modeling Framework for Pairwise and Network Meta-analysis of Randomized Controlled Trials. Med Decis Mak 2013;33(5):607-17.
Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network Meta-Analysis for Decision-Making: Wiley; 2018.
Petropoulou M, Nikolakopoulou A, Veroniki AA, et al. Bibliographic study showed improving statistical methodology of network meta-analyses published between 1999 and 2015. J Clin Epidemiol 2017;82:20-8.
Nikolakopoulou A, Chaimani A, Veroniki AA, Vasiliadis HS, Schmid CH, Salanti G. Characteristics of Networks of Interventions: A Description of a Database of 186 Published Networks. PLoS One 2014;9(1):e86754.
Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159(2):130-7.
Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012;3(2):80-97.
Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med 2013;11:159.
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3(2):98-110.
White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3(2):111-25.
Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013;42(1):332-45.
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8(10):e76654.
White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J 2011;11(2):255-70.
Pesic M, Zivic S, Radenkovic S, Velojic M, Dimic D, Antic S. Comparison between basal insulin glargine and NPH insulin in patients with diabetes type 1 on conventional intensive insulin therapy. Vojnosanit Pregl 2007;64(4):247-52.
Ludemann J, Milek K, Wilhelm B, Segner A, Jaeckel E. Daytime flexible application of Insulin degludec in patients with type 1 diabetes or type 2 diabetes. MMW Fortschr Med. 2014;156 Suppl 3:89-97.
Shestakova MV, Antciferov MV, Mayorov AY, et al. Insulin degludec: a new basal insulin analogue with an ultra-long duration of action. Safety and efficacy in Russian patients with diabetes. Diabetes Mellitus 2015;18(4):130-41.
Segovia Portoles R, Ferrer-Garcia JC, Merino-Torres JF, Penalba MT, Albalat Galera R, Pinon-Selles F. Optimal timing of insulin detemir injection in patients with type 1 diabetes and poor metabolic control. Endocrinol Nutr 2010;57(4):140-6.
Hinneburg I. Insulindegludec, a new option for basal insulin needs. Med Monatsschr Pharm 2012:265-6.
Kobayashi M, Iwamoto Y, Kaku K, Kawamori R, Tajima N. 48-week Randomized Multicenter Open-label Parallel Group Phase 3 Trial to Compare Insulin Detemir and NPH Insulin Efficacy and Safety in Subjects with Insulin Requiring Diabetes Mellitus in a Basal-bolus Regimen. J Jpn Diabetes Soc 2007;50(9):649-63.
Philippo H. A 26-week, Multi-centre, Open-labelled, Randomised, Parallel Efficacy and Safety Comparison of Insulin Detemir and NPH Insulin in Adult Patients with Type 1 Diabetes Mellitus on a Basal-Bolus Regimen Novo Nordisk. 2007. [Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT00595374].
Vaughan K. An Open-Label, Randomized, Multi-center, Parallel-Group Clinical Trial Comparing the Efficacy and Safety of Mylan's Insulin Glargine with Lantus in Type 1 Diabetes Mellitus Patients. 2017. [Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-000747-32/results].
Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53(6):1614-20.
Russell-Jones D, Simpson R, Hylleberg B, Draeger E, Bolinder J. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin Ther 2004;26(5):724-36.
Mathieu C, Hollander P, Miranda-Palma B, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab 2013;98(3):1154-62.
Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012;379(9825):1489-97.
Heise T, Hovelmann U, Nosek L, Hermanski L, Bottcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol 2015;11(8):1193-201.
Koehler G, Heller S, Korsatko S, et al. Insulin degludec is not associated with a delayed or diminished response to hypoglycaemia compared with insulin glargine in type 1 diabetes: a double-blind randomised crossover study. Diabetologia 2014;57(1):40-9.
Iga R, Uchino H, Kanazawa K, et al. Glycemic Variability in Type 1 Diabetes Compared with Degludec and Glargine on the Morning Injection: An Open-label Randomized Controlled Trial. Diabetes Ther 2017;8(4):783-92.
Birkeland KI, Home PD, Wendisch U, et al. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care 2011;34(3):661-5.
Heise T, Bain SC, Bracken RM, et al. Similar risk of exercise-related hypoglycaemia for insulin degludec to that for insulin glargine in patients with type 1 diabetes: a randomized cross-over trial. Diabetes Obes Metab 2016;18(2):196-9.
Lane W, Bailey TS, Gerety G, et al. Effect of Insulin Degludec vs Insulin Glargine U100 on Hypoglycemia in Patients With Type 1 Diabetes: The SWITCH 1 Randomized Clinical Trial. JAMA 2017;318(1):33-44.
Heise T, Norskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: Lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab 2017;19(7):1032-9.
Korsatko S, Deller S, Koehler G, et al. A comparison of the steady-state pharmacokinetic and pharmacodynamic profiles of 100 and 200 U/mL formulations of ultra-long-acting insulin degludec. Clin Drug Investig 2013;33(7):515-21.
Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab 2012;14(9):859-64.
Bailey TS, Pettus J, Roussel R, et al. Morning administration of 0.4U/kg/day insulin glargine 300U/mL provides less fluctuating 24-hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100U/mL in type 1 diabetes. Diabetes Metab 2018;44(1):15-21.
Wirtz VJ, Kaplan WA, Kwan GF, Laing RO. Access to Medications for Cardiovascular Diseases in Low- and Middle-Income Countries. Circulation 2016;133(21):2076-85.
World Health Organization. NCD Global Monitoring Framework. 2019. [Available from: https://www.who.int/nmh/global_monitoring_framework/en/].
Acknowledgements
We thank Becky Skidmore for conducting the literature searches, Jessie McGowan for peer reviewing the MEDLINE search strategy, and Alissa Epworth for running the literature search. We would also like to thank Dr. Kednapa Thavorn for ordering insulins chronologically from oldest to newest according to Health Canada/Federal Drug Administration approval dates, Jane P. Sharpe for abstracting data and assessing study quality, Reid Robson for abstracting data, Amalka De Silva for contacting authors and generating data tables, Krystle Amog for making data tables and formatting the manuscript, and Katrina Chiu for generating figures, making data tables, and formatting the manuscript.
Data and Materials Availability
ACT has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Sharing
The full dataset is available from the corresponding author upon request.
Funding
This systematic review was funded by the Canadian Institutes of Health Research/Drug Safety and Effectiveness Network (CIHR DSEN) MAGIC grant. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation and Quality of Care, the Mary Trimmer Chair in Geriatric Medicine, and a Foundation Grant (Canadian Institutes of Health Research), and ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis.
Author information
Authors and Affiliations
Contributions
ACT conceived and designed the study, helped to obtain funding for the study, screened literature for inclusion, abstracted data from included studies, and drafted and revised the manuscript. HMA coordinated the study, screened the literature search results, abstracted data, resolved discrepancies, cleaned data, and drafted and revised the manuscript. JA screened the literature search results, abstracted data, resolved discrepancies, cleaned data, and edited the manuscript. ZB, MR conducted analysis and interpretation of the data. BP provided input for data analysis, conducted interpretation of the data, and edited sections of the manuscript. ND screened the literature search results, abstracted data, conducted quality assessment, and resolved discrepancies. PAK screened the literature search results, abstracted data, conducted the quality assessment, helped with data cleaning, and drafted sections of the manuscript. VN abstracted data, conducted the quality assessment, and helped with data cleaning. MG, FY, screened the literature search results, abstracted data, and conducted the quality assessment. JDI screened the literature search results and abstracted data. AAV conceived and designed the study and helped to obtain funding for the study. LM provided input. CY provided clinical input. SES conceived and designed the study, obtained the funding, and provided clinical input.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Disclaimer
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lorenzo Moja works in the Department of Essential Medicines and Health Products, World Health Organization, Geneva.
Rights and permissions
About this article
Cite this article
Tricco, A.C., Ashoor, H.M., Antony, J. et al. Comparative Efficacy and Safety of Ultra-Long-Acting, Long-Acting, Intermediate-Acting, and Biosimilar Insulins for Type 1 Diabetes Mellitus: a Systematic Review and Network Meta-Analysis. J GEN INTERN MED 36, 2414–2426 (2021). https://doi.org/10.1007/s11606-021-06642-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-06642-7