Abstract
Background
Mailed fecal immunochemical testing (FIT) can increase colorectal cancer (CRC) screening rates, including for vulnerable patients, but its cost-effectiveness is unclear.
Objective
We sought to examine the effectiveness and cost-effectiveness of the initial cycle of our mailed FIT program from November 2017 to July 2019 in a federally qualified health center (FQHC) system in Central Texas.
Design
Single group intervention and economic analysis
Participants
Eligible patients were those ages 50–75 who had been seen recently in a system practice and were not up to date with screening.
Intervention
The program mailing packet included an introductory letter in plain language, the FIT itself, easy to read instructions, and a postage-paid lab mailer, supplemented with written and text messaging reminders.
Main Measures
We measured effectiveness based on completion of mailed FIT and cost-effectiveness in terms of cost per person screened. Costs were measured using detailed micro-costing techniques from the perspective of a third-party payer and expressed in 2019 US dollars. Direct costs were based on material supply costs and detailed observations of labor required, valued at the wage rate.
Key Results
Of the 22,838 eligible patients who received program materials, mean age was 59.0, 51.5% were female, and 43.9% were Latino. FIT were successfully completed by 19.2% (4395/22,838) patients at an average direct cost of $5275.70 per 500-patient mailing. Assuming completed tests from the mailed intervention represent incremental screening, the direct cost per patient screened, compared with no intervention, was $54.83. Incorporating start-up and indirect costs increases total costs to $7014.45 and cost per patient screened to $72.90. Alternately, assuming 2.5% and 5% screening without the intervention increased the direct (total) cost per patient screened to $60.03 ($80.80) and $67.05 ($91.47), respectively.
Conclusions
Mailed FIT is an effective and cost-effective population health strategy for CRC screening in vulnerable patients.
Similar content being viewed by others
BACKGROUND
Colorectal cancer (CRC) remains the second leading cause of cancer mortality in the USA.1 Screening is effective in reducing morbidity and mortality but is underutilized, especially in vulnerable patients such as uninsured and Latinx patients, many of whom receive care in federally qualified health centers (FQHC).2–3 More effective interventions are needed to help reduce the burden of colorectal cancer, particularly for vulnerable patients.
Mailed stool-based fecal immunochemical testing-based screening interventions (hereafter “mailed FIT”) have been shown to increase colorectal cancer screening rates, are scalable, and have great promise for underserved and safety net populations.4 They are designed to overcome several barriers (e.g., need for transportation, need to take time off of work) that may disproportionately affect those with limited income or resources.
The effectiveness of mailed FIT has been demonstrated in prior studies, including several in safety net populations.4,5,6,7 Most programs consist of an initial contact (primer) to notify the patient that outreach will follow and to confirm the correct address and screening eligibility; the main mailing of the FIT, along with easy to read instructions for completion and a pre-addressed mailer to return the FIT; one or more reminders (text, mail, or phone) to help ensure completion; and (in some cases) navigation assistance to help patients with positive tests receive follow-up colonoscopy.4,5,6,7 Generally, previously evaluated mailed FIT programs directed to unselected populations (i.e., without having been previously recruited or formally enrolled in a program) have achieved test completion rates of 20–25%.4
Data on the cost-effectiveness of mailed FIT is limited.4 More intensive interventions, because they can devote greater effort to overcoming barriers through additional reminder calls for example, are generally more effective, but also have greater costs. As the cost per person of the intervention increases, the program’s ability to serve additional patients may become limited, especially in the context of fixed overall program budgets. To derive the greatest benefit from mailed FIT programs, we must optimize their cost-effectiveness. We sought to measure the effectiveness and cost-effectiveness of a mailed FIT program in a safety net urban health system.
METHODS
Overview
We initiated a mailed FIT program for age-eligible patients in a large safety net, federally qualified health center (FQHC) system in Central Texas, with 24 locations. The system did not employ any outreach or reminder systems to prompt screening, and relied on opportunistic approaches for preventive care.
Eligibility
We included patients aged 50–75 years who were not up to date with CRC screening (no stool test in the past 12 months, sigmoidoscopy in the past 5 years, or colonoscopy in the past 10 years based on information available in patients’ medical records), and who had at least one visit in the previous 12 months or 2 visits in the previous 24 months. Patients with a documented personal history of CRC in the medical record were excluded. At the beginning of the program in November 2017, chart review identified 20,146 eligible patients who were not up to date with screening. At that time, data from the FQHC system suggested that only 18.4% of the patients in the 50–75 age range were up to date with CRC screening.
Patient eligibility was updated monthly, with new patients becoming eligible as they entered the system or aged into the screening cohort and others aging out. We consider here only first outreach mailings. The effectiveness and cost-effectiveness of serial outreach in subsequent years will be examined in a future analysis.
Intervention
The intervention consisted of a mailing packet that included an introductory letter in plain language, the FIT itself (Polymedco OC–Auto FIT ™), easy to read instructions with pictures, and a postage-paid lab mailer. We also included a “records update card” for patients to indicate prior screening that was not documented in the patient’s medical record. All materials were provided in both English and Spanish. Importantly, the FIT and any subsequent colonoscopy were provided without charge for patients without insurance through the program and this information was included in the mailing materials.
Packets were mailed weekly in waves of 300–550, from a central location, directly to patients’ homes. If patients did not complete the FIT, they received a text message reminder 2.5 weeks later and a reminder letter 5 weeks after initial mail out, unless they opted out of future contacts or had previously declined to enroll in text message receipt. We chose to employ text and mail reminders rather than phone calls to keep the cost of the intervention manageable.
Because those who were included in the program and received the intervention had not been screened recently, and the rate of in-clinic screening was very low and relatively flat prior to the mailed FIT program, our base scenario assumed patients would not have been screened without the program. We examined this assumption in sensitivity analyses described below.
Outcomes
The primary outcome measured was the proportion of completed FIT. FIT were counted as successfully completed if our lab received the FIT and provided a lab result to the clinic and program staff. We did not set a time limit for completion of the test, but of those who completed the FIT, 94.7% were completed within 3 months and 98.0% within 6 months. In a secondary analysis, we also counted as successful those patients who returned the records update card stating the date of the patient’s recent stool test or colonoscopy. FITs that were returned but could not be processed due to mail delay or laboratory error were counted separately. We also measured the outcomes of patients with positive FITs, including timely completion of colonoscopy and findings at colonoscopy.
The University of Texas at Austin Office of Research Support and Compliance reviewed the protocol and judged the work not to constitute human subjects research, as it evaluated the delivery of a recommended preventive service.
Returning FIT Results
Patients with negative FIT results were informed of their results in writing and had their results entered into the electronic health record and our program database.
Patients with positive (abnormal) FIT results received a phone call followed by a letter recommending that the patient schedule a colonoscopy with the help of our bilingual patient navigator. The navigator provided additional education about positive results, helped the patient schedule a pre-evaluation and the colonoscopy procedure itself, and helped troubleshoot any problems that arose or threatened adherence.
All patients found to have CRC were referred for appropriate treatment. Patients in whom polyps were identified and removed were entered into a surveillance database for recall at the appropriate evidence-based surveillance interval.
Costing
Our analysis followed the best practices recommended in the CHEERS reporting guidelines (see online Supplementary Information for checklist).8 The detailed cost analysis estimated the costs of start-up activities (e.g., training, equipment, database development) and on-going activities. On-going direct costs included per patient costs of supplies, FIT processing, and labor associated with mailer assembly, reminder and results letters, updates to the electronic health record, positive results calls, and navigation to colonoscopy. On-going indirect costs included labor associated with administration, management, and data quality assessment. All labor was valued at the wage rate and included fringe. Non-labor unit costs were obtained from program invoices. Activity times were obtained through detailed observation of and/or interviews with personnel performing each task. All costs were estimated from the perspective of a third-party payer and reported in 2019 US dollars.
To reflect usual operations as they occurred in the study, costs are presented per batch of 500 mailers (Table 1). Although most of the items presented in the detailed cost analysis are straightforward, two require explanation. Start-up costs and annual indirect costs were prorated to 500-mailer batches assuming 13,050 mailers per year (i.e., (22,838 mailers during study/21 study months) * 12 months). Before prorating, start-up costs were converted to an equivalent annual cost following Drummond et al.9, assuming a 3% discount rate (as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine10) and three years of useful life for all start-up activities and equipment.
Cost-effectiveness
Cost effectiveness was assessed using incremental cost-effectiveness ratios (ICERs). The ICER for our base case was defined as the cost per additional patient screened for the intervention compared with no intervention, assuming no screening in this group under usual care. The cost per additional patient screened was determined by dividing the cost per 500-person mailing by the additional number of returned tests.
We prospectively defined our goal for cost-effectiveness to be $50 (direct cost) per additional patient screened, based on prior studies4 and one analysis that found strong cost-effectiveness at that level of cost per additional patient screened.11 We based our goal on direct costs because the start-up and indirect costs would be minimized as the program grew and matured.
Sensitivity Analyses
To determine how our results would change had the program been implemented under alternative realistic conditions, we conducted sensitivity analysis in which we varied (a) annual output of mailers (to reflect potential variation in clinic size), (b) unit costs (labor and non-labor, to reflect potential geographic variation in costs should the program be implemented elsewhere), (c) mean time navigating to colonoscopy, and (d) baseline screen rate (to account for the possibility that some patients might screen without the program). Unless stated otherwise, the base scenario is based on the actual costs (i.e., resource utilizations multiplied by unit costs) as occurred in the program. In all scenarios except those that varied the baseline screen rate, the ICER is identical to the average cost effectiveness ratio.
RESULTS
Patient Demographics
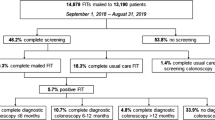
Demographic data for the full sample of 22,838 participants who became eligible for the program during the study time period and were mailed FIT are shown in Table 2. Mean age was 59.0 years. Almost half identified as Latinx, and nearly half did not have traditional private or government health insurance.
Effectiveness
Of 22,838 eligible patients who were mailed program materials, 4395 completed FIT successfully (19.2%). Additionally, 423 health information cards were returned providing updated information on screening status. When these responses are included, it brings the successful response total to 4818, or 21.1%.
Finally, 255 FIT (5.5% of all returned FIT) were returned but could not be processed in the lab due to mail delays or collection errors.
Costs
Table 1 provides a detailed breakdown of the start-up and on-going costs in the base scenario, while Table 3 presents the incremental cost-effectiveness ratios and results of the sensitivity analyses. The direct cost of the program per 500 patients was estimated to be $5275.70 based on direct patient related activities, materials, and FIT processing fee. Postage was the largest component of the direct cost (38.0%), followed by the FIT processing fees (36.5%), labor (20.6%), and supplies (4.9%). Incorporating start-up and indirect costs increased the total cost to $7014.45 per 500-person mailing.
Cost-effectiveness—Base Case
The direct cost per patient screened was $54.83 ($5275.70/96.22 tests completed for each 500 person mailing) and the total cost per patient screened was $72.90, based on the FIT completion rate of 19.2% and assuming no screening in the absence of the intervention.
Sensitivity Analyses
As seen in Table 3, the direct cost per person screened ranged from $43.86 to $67.05 across the sensitivity scenarios, and the total cost per patient screened ranged from $58.32 to $91.47. Assuming 2.5% and 5% screening without the intervention increased the direct (total) cost per patient screened to $60.03 ($80.80) and $67.05 ($91.47), respectively.
Positive FIT Results
Overall, 258 of 4395 patients (5.9%) who successfully completed FIT had positive results. Of the 258 patients with positive FIT results, 195 (76%) completed colonoscopy.
For the 195 patients who completed colonoscopy, 8 had cancers detected, 76 had adenomas (including 6 with high-grade dysplasia), 24 hyperplastic polyps, and 68 had normal results. For 19 patients, we did not have complete records of their colonoscopy findings.
DISCUSSION
Our mailed FIT program successfully engaged over 20% of patients from our local FQHC system who were overdue for CRC screening, either by helping them become up to date with screening (19.2%) or by identifying them as having been screened outside of the system (and hence not requiring further outreach: 1.9%). The direct cost per patient screened, based on rigorous micro-costing, was approximately $55 and our total cost was $72.90. These values were slightly above our initial goal but similar or somewhat lower than values obtained in previous mailed FIT programs4 and consistent with previous estimates of costs for widespread mailed FIT program implementation.12
We were successful in reaching a highly vulnerable patient population served by a large federally qualified health center (FQHC) system. Screening rates in FQHC settings are under 50% nationally,13 and represent an important opportunity for efforts to increase screening and reduce disparities in colorectal cancer incidence and mortality. The mailed FIT program was designed to complement the health system’s efforts to increase screening through office-based visits. To sustain or magnify the additional screening observed will require ongoing outreach and adherence to mailed FIT and additional efforts to reach unscreened patients during visits.
We purposefully chose to develop a low-cost, widely scalable program in order to improve the chances of sustainability over time. As such, we did not include “live” reminder calls to patients from program or clinic staff. While such calls may improve adherence, they add significant costs to the program.11 Instead, we relied on text messaging and mailed reminders, which are lower cost and scalable but produce lower levels of response compared with “live” calls.
In contrast, we devoted significant additional resources to providing bilingual patient navigation for patients after positive tests, recognizing that the barriers to completing follow-up colonoscopy may be particularly important in our low-income, frequently uninsured patients, many of whom spoke Spanish as their preferred language. We were able to successfully navigate over 75% of patients to colonoscopy, many of whom faced significant socioeconomic barriers to care. Successful completion of high-quality colonoscopy is critical to the overall effectiveness of FIT screening programs. Not surprisingly, a high proportion of those completing colonoscopy had important findings of cancer or adenomas.
Our costs and costs per patient screened were fairly robust to changes in key parameters. As expected, increasing annual output had no effect on direct costs per person but reduced the prorated start-up and indirect costs substantially; conversely, increasing the baseline screening rate reduced the net effect of the program and consequently increased the cost per additional patient screened. Further, because we are only measuring the costs of screening implementation, we did not assess or capture any of the potential economic savings from reducing costs of treatment through preventing cases and reducing late-stage disease. More extensive cost-effectiveness analyses have suggested that mailed FIT remains cost-effective ($37,500 per quality adjusted life year gained) at costs per patient screened greater than those observed in our study.11
Our effectiveness and cost-effectiveness compare favorably with other mailed FIT programs reported in the literature. A study done in safety net clinics in Washington state found a cost per completed FIT of $39.81, based on an initial mailing, two reminder calls and a reminder letter.14 However, while this study included both direct and indirect costs in their estimate, they apparently did not include the cost of the FIT cards themselves, or the cost of completed FIT processing. Adding these costs would nearly double the total program cost. In addition, their cost estimates were developed retrospectively and may have been subject to recall bias.
Somsouk and colleagues used a “primer” postcard, followed by the FIT mailing and reminder phone calls, and found a cost per additional patient screened of $112 based on a 20 percentage point increase in screening compared with usual care.11 Sequist and colleagues found a cost per additional patient screened of $94 for a mailed intervention with a mailed reminder, based on an increase in screening of 6% points beyond that observed in a control group.15 Meenan and colleagues found a 3.6% point increase in screening with mailed FIT outreach in their 26-clinic trial in the Pacific Northwest, with a cost per additional patient screened of $483.16 Direct comparison among these studies is challenging based on differences in costing methods, study design, and settings. Future work is needed to better define best practices for measuring costs of different program components.
Strengths of our study include a large sample of diverse patients and rigorous micro-costing methods. We also have provided extensive methodological detail explaining the assumptions underlying the cost analysis to enable both practitioners and researchers to model alternative implementation scenarios, and our findings are supported by sensitivity analyses on key parameters. Further, we included patient navigation as a means of ensuring high rates of colonoscopy follow-up, a necessary condition for reducing CRC incidence and mortality.
However, our program does have some limitations. We did not include a control group, so we cannot be sure that all of our patients reached through mailed FIT would not have been screened otherwise. That said, the stable, low clinic-based screening rates in this system argue against an important secular trend and, in any case, we conducted sensitivity analysis on this assumption. Finally, our program was implemented in the context of a very low baseline screening rate and a highly Latinx, often uninsured, urban population that was afforded access to screening and colonoscopy follow-up without associated costs; whether similar improvements would be observed in different populations is unclear and should be studied.
With these limitations in mind, we nevertheless find mailed FIT to be an effective and cost-effective program to provide CRC screening in a vulnerable population served in an FQHC system. Assuming that most of the FIT screening represents new screening, it also offers the opportunity to reduce CRC-related morbidity and mortality and thus improve health equity. We plan to expand and implement a similar program in other nearby health systems, including ones with more rural coverage. We will also continue to offer FIT and follow participants and non-participants over time, to better understand patterns of screening adherence longitudinally.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
American Cancer Society. Cancer Prevention & Early Detection Facts & Figures 2017-2018.
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. https://doi.org/10.3322/caac.21395
Gupta S, Coronado GD, Argenbright K, et al. Mailed fecal immunochemical test outreach for colorectal cancer screening: Summary of a Centers for Disease Control and Prevention-sponsored Summit. CA Cancer J Clin. 2020;70(4):283-298. https://doi.org/10.3322/caac.21615
Jager M, Demb J, Asghar A, et al. Mailed outreach is superior to usual care alone for colorectal cancer screening in the USA: a systematic review and meta-analysis. Dig Dis Sci. 2019;64(9):1-8. https://doi.org/10.1007/s10620-019-05587-6
Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer. 2016;122(3):456-463.
Coronado GD, Petrik AF, Vollmer WM, et al. Effectiveness of a mailed colorectal cancer screening outreach program in community health clinics: the STOP CRC cluster randomized clinical trial. JAMA Inter Med. 2018;178(9):1174-1181.
Husereau D, Drummond M, Petrou S, et al. ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231-50. https://doi.org/10.1016/j.jval.2013.02.002.
Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programs. 4th ed. Oxford University Press, Oxford, UK. 2015.
Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. 2nd ed. Oxford University Press, Oxford, UK. 2017.
Somsouk M, Rachocki C, Mannalithara A, et al. Effectiveness and cost of organized outreach for colorectal cancer screening: a randomized, controlled trial. J Natl Cancer Inst. 2020;112:305-313.
Guy GP Jr, Richardson LC, Pignone MP, Plescia M. Costs and benefits of an organized fecal immunochemical test-based colorectal cancer screening program in the United States. Cancer. 2014;120:2308-2315.
National Colorectal Cancer Roundtable. Colorectal Cancer Screening Rates Reach 44.1% in FQHCs in 2018. Available at: https://nccrt.org/colorectal-cancer-screening-rates-reach-44-1-in-fqhcs-in-2018/. Accessed August 2020.
Kemper KE, Glaze BL, Eastman CL, et al. Effectiveness and cost of multilayered colorectal cancer screening promotion interventions at federally qualified health centers in Washington State. Cancer. 2018;124:4121-4129.
Sequist TD, Franz C, Ayanian JZ. Cost-effectiveness of patient mailings to promote colorectal cancer screening. Med Care.. 2010;48(6):553-7. https://doi.org/10.1097/MLR.0b013e3181dbd8eb.PMID:20473196
Meenan RT, Coronado GD, Petrik A, Green BB. A cost-effectiveness analysis of a colorectal cancer screening program in safety net clinics. Prev Med. 2019;120:119-125. https://doi.org/10.1016/j.ypmed.2019.01.014. Epub 2019 Jan 24. PMID: 30685318
Acknowledgements
The authors wish to thank Angelica Ferrandino, Madison Gove, Jaclyn Le, Hanna Munnin, Felipe Hernandez, Alice Kelly, Zoe Cook, Eda Baykal-Caglar, and the CommUnityCare network for their support of this project.
Funding
Cancer Prevention and Research Institute of Texas (CPRIT) funded the program (PP170082). The content of this manuscript solely reflects the authors’ views and not those of the funding agency or the authors’ institutional affiliates.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentations
Society of General Internal Medicine, May 9, 2019
Supplementary Information
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Pignone, M., Lanier, B., Kluz, N. et al. Effectiveness and Cost-effectiveness of Mailed FIT in a Safety Net Clinic Population. J GEN INTERN MED 36, 3441–3447 (2021). https://doi.org/10.1007/s11606-021-06691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-06691-y



