Abstract
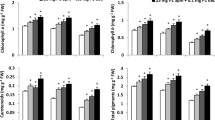
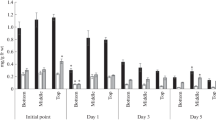
This paper presents a study of the metabolic response (dark respiration intensity, photosystem II efficiency, metabolic activity) and the yield of barley treated with 24-epibrassinolide and subjected to high-temperature stress. Transport of exogenously applied 24-epibrassinolide in barley and changes in the profile of brassinosteroids that may occur in tissues after 24-epibrassinolide application were also studied. The water solution of 24-epibrassinolide (0.005 and 0.25 mg dm−3) was applied via infiltration of the first and second leaves of 12-day-old seedlings. Control plants were treated with water solution of hormone solvent (ethanol). Fifteen-day-old plants were subjected to high-temperature stress (42°C for 3 h). The influence of hormone treatment and stress conditions was investigated in the first and second leaves based on measurements of PSII efficiency. The aftereffect of plant treatment was investigated in the seventh leaf (measurements of PS II efficiency, dark respiration intensity, metabolic activity). The transport efficiency of 24-epibrassinolide exogenously applied to the first and second leaves, as well as the profile of other brassinosteroids, was also measured on the seventh leaf. Finally, yield formation was estimated. 24-epibrassinolide showed protective action, which manifested itself in the improved functioning of PSII, but this was observed in case of higher hormone concentration and only for the first, older leaf. The PSII efficiency of the seventh leaf was similar in plants treated with brassinosteroid and in the control plants, whereas the respiration intensity and metabolic activity decreased in plants previously treated with higher concentration of 24-epibrassinolide. The use of a higher hormone concentration at the seedling phase ultimately resulted also in lower crop yield. Brassinosteroids—brassinolide and castasterone—were detected in barley leaves. 24-epibrassinolide was found only in trace amounts in control plants. Its exogenous application directly to the apoplast of the first and second leaves resulted in an increase in the 24-epibrassinolide content in the seventh leaf, but did not depend on whether a high or low concentration had been applied to the plants.

Similar content being viewed by others
References
Ali Q, Athar H, Ashraf M (2008) Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul 56:107–116
Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Sci 171:382–388
Asakawa S, Abe H, Nishikawa N, Natsume M, Koshioka M (1996) Purification and identification of new acyl-conjugated teasterons in lily pollen. Biosci Biotechnol Biochem 60:1416–1420
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bajguz A, Tretyn A (2003) The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62:1027–1046
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel K-H, Schäfer P, Schwarczinger I, Zuccaro A, Skoczowski A (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 180:501–510
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Braun P, Wild A (1984) The influence of brassinosteroid on growth and parameters of photosynthesis of wheat and mustard plants. J Plant Physiol 116:189–196
Brown KW, Thomas JC (1980) The influence of water stress preconditioning on dark respiration. Physiol Plant 49:205–209
Chaitanya KV, Sundar D, Masilamani S, Reddy AR (2002) Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul 36:175–180
Criddle R, Hansen L (1999) Calorimetric methods for analysis of plant metabolism. In: Kemp RB (ed) Handbook of thermal analysis and calorimetry. Elsevier, Amsterdam, pp 711–763
Criddle RS, Hansen LD, Breidenbach RW, Ward MR, Huffake RC (1989) Effects of NaCl on metabolic heat evolution rates by barley roots. Plant Physiol 90:53–58
Dash S, Mohanty N (2002) Response of seedlings to heat-stress in cultivars of wheat: growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity source. J Plant Physiol 159:49–59
Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342
Dhaubhadel S, Browning KS, Gallie DR, Krishna P (2002) Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J 29:681–691
El-Shintinawy F, Ebrahim MKH, Sewelam N, El-Shourbagy MN (2004) Activity of photosystem 2, lipid peroxidation, and the enzymatic antioxidant protective system in heat shocked barley seedlings. Photosynthetica 42:15–21
Fariduddin Q, Ahmad A, Hayat S (2003) Photosynthetic response of Vigna radiata to pre-sowing seed treatment with 28-homobrassinolide. Photosynthetica 41:307–310
Fariduddin Q, Hasan SA, Ali B, Hayat S, Ahmad A (2008) Effect of modes of application of 28-homobrassinolide on mung bean. Turk J Biol 32:17–21
Grove MD, Spencer GF, Rohwedder WK, Mandawa N, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson JL, Cook JC (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Hansen LD, Macfarlane C, McKinnon N, Smith BN, Criddle RS (2004) Use of calorespirometric ratios, heat per CO2 and heat per O2, to quantify metabolic paths and energetics of growing cells. Thermochim Acta 422:55–61
Hayat S, Ahmad A, Mobin M, Hussain A, Fariduddin Q (2000) Photosynthetic rate, growth, and yield of mustard plants sprayed with 28-homobrassinolide. Photosynthetica 38:469–471
Heckathorn SA, Downs CA, Sharkey TD, Coleman JS (1998) The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol 116:439–444
Janeczko A, Swaczynová J (2010) Endogenous brassinosteroids in wheat treated with 24-epibrassinolide. Biol Plant 54:477–482
Janeczko A, Kościelniak J, Pilipowicz M, Szarek-Łukaszewska G, Skoczowski A (2005) Protection of winter rape photosystem II by 24-epibrassinolide under cadmium stress. Photosynthetica 43:293–298
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007a) Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358
Janeczko A, Tóbias I, Skoczowski A, Dubert F, Gullner G, Barna B (2007b) Bacterial infection and pretreatment with 24-epibrassinolide markedly affect the heat emission and membrane permeability of rape cotyledons. Thermochim Acta 458:89–91
Janeczko A, Biesaga-Kościelniak J, Dziurka M (2009) 24-Epibrassinolide modifies seed composition in soybean, oilseed rape and wheat. Seed Sci Technol 37:625–637
Janeczko A, Biesaga-Kościelniak J, Oklešťková J, Filek M, Dziurka M, Szarek-Łukaszewska G, Kościelniak J (2010) Role of 24-epibrassinolide in wheat production: physiological effects and uptake. J Agron Crop Sci 196:311–321
Jiang GM, Hao NB, Bai KZ, Zhang QD, Sun JZ, Guo RJ, Ge QY, Kuang TY (2000) Correlation between variables of gas exchange and yield potential in different winter wheat cultivars. Photosynthetica 38:227–232
Kolbe A, Schneider B, Porzel A, Schmidt J, Adam G (1995) Acyl-conjugated metabolites of brassinosteroids in cell suspension cultures of Ornithopus sativus. Phytochemistry 38:633–636
Korotaeva NE, Antipina AI, Grabelnykh OI, Varakina NN, Borovskii GB, Voinikov VK (2001) Mitochondrial low-molecular weight heat-shock proteins and the tolerance of cereal mitochondria to hyperthermia. Russ J Plant Physiol 48:798–803
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297
Lambers H, Ribas-Carbó M (2005) Advances in photosynthesis and respiration—plant respiration: from cell to ecosystem. Springer, Dordrecht
Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N (2002) Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol Biol 48:667–681
Mandava NB (1988) Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol 39:23–52
Mazorra LM, Núñez M (2000) Brassinosteroid analogues differentially modify peroxidase activity, superoxide dismutase activity and protein content in tomato seedlings. Cultiv Trop 21:29–33
Mazorra LM, Núñez M, Hechavarria M, Coll F, Sanchez-Blanco MJ (2002) Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol Plant 45:593–596
Miller-Morey JS, Van Dolah FM (2004) Differential expression of stress proteins, antioxidant enzymes, and photosynthetic efficiency to physiological stresses in the Florida red tide dinoflagellate, Karenia brevis. Comp Biochem Physiol 138:493–505
Nishikawa N, Toyama S, Shida A, Futatsuya F (1994) The uptake and the transport of 14C-labeled epibrassinolide in intact seedlings of cucumber and wheat. J Plant Res 107:125–130
Nishikawa N, Shida A, Toyama S (1995) Metabolism of 14C-labeled epibrassinolide in intact seedlings of cucumber and wheat. J Plant Res 108:65–69
Ortiz C, Cardemil L (2001) Heat-shock responses in two leguminous plants: a comparative study. J Exp Bot 52:1711–1719
Płażek A, Rapacz M (2000) The intensity of respiration and heat emission from seedlings of Festuca pratensis (Hud.) and Hordeum vulgare L. during pathogenesis caused by Bipolaris sorokiniana (Sacc.) Shoem. Acta Physiol Plant 22:25–30
Pociecha E, Janeczko A (2008) Antioxidant enzymes activity and development of barley after high temperature stress—impact of 24-epibrassinolide. Zesz Probl Post Nauk Rol 524:83–93
Ramraj VM, Vyas BN, Godrej NB, Mistry KB, Swami BN, Singh N (1997) Effects of 28-homobrassinolide on yields of wheat, rice, groundnut, mustard, potato and cotton. J Agric Sci 128:405–413
Rapacz M, Żur I (1996) The use of microcalorimetric measurements in the investigations on the effect of various cold-acclimation methods on winter rape (Brassica napus var. oleifera) callus growth and frost resistance. In: Grzesiak S, Miszalski Z (eds) Ecophysiological aspects of plant responses to stress factors. ZFR PAN, Krakow, pp 483–495 (in Polish with abstract in English)
Sairam RK (1994) Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul 14:173–181
Sarhan F, Perras M (1987) Accumulation of a high molecular weight protein during cold hardening of wheat (Triticum aestivum L.). Plant Cell Physiol 28:1173–1179
Sembdner G, Atzorn R, Schneider G (1994) Plant hormone conjugation. Plant Mol Biol 26:1459–1481
Shahbaz M, Ashraf M, Athar H (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul 55:51–64
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119
Skoczowski A, Janeczko A, Gullner G, Tóbias I, Kornaś A, Barna B (2010) Response of brassinosteroid treated oilseed rape cotyledons to infection with wild type and HR-mutant of Pseudomonas syringe or with P. florescence. J Therm Anal Cal (in press) doi:10.1007/s10973-010-1204-z
Smith BN, Lytle CM, Hansen LD (1995) Predicting plant growth rates from dark respiration rates: an experimental approach. In: Roundy BA, McArthur ED, Haley JS, Mann DK (eds) Proceedings: wildland shrub and arid land restoration symposium 1993 October 19–21; Las Vegas, NV. Gen. Tech. Rep. INT-GTR-315. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station, pp 243–245
Smith BN, Criddle RS, Hansen LD (2000) Plant growth, respiration and environmental stress. J Plant Biol 27:89–97
Srivastava LM (2002) Brassinosteroids, chap 9. In: Srivastava LM (ed) Plant growth and development. Hormones and environment. Academic Press/Elsevier, USA, pp 212–213
Stokłosa A, Janeczko A, Skoczowski A, Kieć J (2006) Isothermal calorimetry as a tool for estimating resistance of wild oat (Avena fatua L.) to aryloxyphenoxypropionate herbicides. Thermochim Acta 441:203–206
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterise and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor & Francis, London, pp 445–483
Swaczynová J, Novák O, Hauserová E, Funksová K, Šíša M, Strnad M (2007) New techniques for estimation of naturally occurring brassinosteroids. J Plant Growth Regul 26:1–14
Symons GM, Ross JJ, Jager CE, Reid JB (2008) Brassinosteroid transport. J Exp Bot 59:17–24
Tambussi EA, Nogues S, Ferrio P, Voltas J, Araus JL (2005) Does higher yield potential improve barley performance in Mediterranean conditions? A case study. Field Crop Res 91:149–160
Vardhini BV, Rao SSR (1998) Effect of brassinosteroids on growth, metabolite content and yield of Arachis hypogaea. Phytochemistry 48:927–930
Vardhini BV, Rao SSR (2002) Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 61:843–847
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42:579–620
Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410:380–383
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 5:1135–1143
Zullo MAT, Adam G (2002) Brassinosteroid phytohormones: structure, bioactivity and applications. Braz J Plant Physiol 14:83–121
Zullo M, Kohout L (2004) Semisystematic nomenclature of brassinosteroids. Plant Growth Regul 42:15–28
Acknowledgments
This study was supported by the European Union CROPSTRESS Program, Project QLK5-CT-2002-30424, by GAAS CR (IAA 400550801) and Grant MSM 6198959216. The authors would like to thank Dr Balázs Barna (Plant Protection Institute, Hungarian Academy of Sciences) for the method of leaf infiltration and Dr Ondrej Novak for help with HPLC–MS/MS measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Janeczko, A., Oklešťková, J., Pociecha, E. et al. Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. Acta Physiol Plant 33, 1249–1259 (2011). https://doi.org/10.1007/s11738-010-0655-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0655-y