Abstract
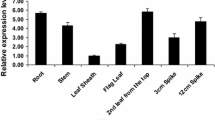
Plant chloride channels (CLCs) localize to the plasma and organellar membranes; these channels play pivotal roles in the modulation of ion homeostasis and cell turgor. Recent studies have shown that the expression of CLCs is involved in plant responses to environmental stress. Here, we examined the rice (Oryza sativa) tonoplast-localized channel OsCLC1. OsCLC1 is preferentially expressed in roots, and therefore, we generated transgenic rice with root-specific overexpression of OsCLC1 (RCc3::OsCLC1). We also identified a T-DNA mutant line that lacks expression of OsCLC1 (osclc1). We found that RCc3::OsCLC1 rice plants showed increased tiller number and grain yield, whereas the osclc1 plants exhibited decreased tiller number and grain yield, compared with wild type. These observations suggest that expression of OsCLC1 affects rice growth and productivity. Furthermore, RCc3::OsCLC1 plants showed enhanced drought tolerance, leading to increased grain yield compared to wild-type plants grown under the same conditions. By contrast, osclc1 mutants exhibited reduced drought tolerance and productivity compared to wild-type plants. When expression of OsCLC1 was analyzed in drought, jasmonic acid (JA) or abscisic acid (ABA)-treated rice, expression of OsCLC1 was preferentially upregulated in roots in response to drought and JA, and was preferentially upregulated in shoots in response to ABA. Together with the finding that expression of OsCLC1 is positively correlated both with expressions of OsDREB1A and OsbHLH148, key transcription factors in drought and JA responses, respectively, these results suggest that OsCLC1 regulates drought tolerance in rice and JA signaling is involved in this process.







Similar content being viewed by others
References
Ahmad P, Rasool S, Gul A, Sheikh SA, Akram NA, Ashraf M, Kazi A, Gucel S (2016) Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci 7:813
Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2014) Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol Rep 8:279–293
Baetz U, Eisenach C, Tohge T, Martinoia E, De Angeli A (2016) Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol 172:1167–1181
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Chen J-Q, Meng X-P, Zhang Y, Xia M, Wang X-P (2008) Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol Lett 30:2191–2198
Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15:532–534 (536–537)
Christmann A, Moes D, Himmelbach A, Yang Y, Tang Y, Grill E (2006) Integration of abscisic acid signalling into plant responses. Plant Biol 8:314–325
Cruz de Carvalho R, Catalá M, Marques da Silva J, Branquinho C, Barreno E (2012) The impact of dehydration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Ann Bot 110:1007–1016
De Angeli A, Monachello D, Ephritikhine G, Frachisse J, Thomine S, Gambale F, Barbier-Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442:939–942
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J 33:751–763
Fu J, Wu H, Ma S, Xiang D, Liu R, Xiong L (2017) OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front plant sci 8:2108
Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelièvre F, Courtial B, Barbier-Brygoo H, Maurel C (2000) Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J 21:259–267
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Jentsch TJ (2008) CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43:3–36
Jeong JS, Kim YS, Redillas MC, Jang G, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, Kim JK (2013) OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol J 11:101–114
Jossier M, Kroniewicz L, Dalmas F, Le Thiec D, Ephritikhine G, Thomine S, Barbier-Brygoo H, Vavasseur A, Filleur S, Leonhardt N (2010) The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J 64:563–576
Kim EH, Kim YS, Park S-H, Koo YJ, Choi YD, Chung Y-Y, Lee I-J, Kim J-K (2009) Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol 149:1751–1760
Lee D-K, Jung H, Jang G, Jeong JS, Kim YS, Ha S-H, Do Choi Y, Kim J-K (2016a) Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol 172:575–588
Lee D-K, Park S-H, Seong S-Y, Kim YS, Jung H, Choi YD, Kim J-K (2016b) Production of insect-resistant transgenic rice plants for use in practical agriculture. Plant Biotechnol Rep 10:391–401
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Biol 49:199–222
Li X, Cheng X, Liu J, Zeng H, Han L, Tang W (2011) Heterologous expression of the Arabidopsis DREB1A/CBF3 gene enhances drought and freezing tolerance in transgenic Lolium perenne plants. Plant Biotechnol Rep 5:61–69
Lim H, Hwang HJ, Kim AR, Cho MH, Ji H, Kim CK, Ji SU, Cho JI, Park SC, Lee G-S (2016) A simple, rapid and systematic method for the developed GM rice analysis. Plant Biotechnol Rep 10:25–33
Marmagne A, Vinauger-Douard M, Monachello D, De Longevialle AF, Charon C, Allot M, Rappaport F, Wollman F-A, Barbier-Brygoo H, Ephritikhine G (2007) Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J Exp Bot 58:3385–3393
Martinoia E, Maeshima M, Neuhaus HE (2006) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58:83–102
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Passioura JB, Guo J, Chazen O, Cramer GR (2000) Water relations and leaf expansion: importance of time scale. J Exp Bot 51:1495–1504
Nakamura A, Fukuda A, Sakai S, Tanaka Y (2006) Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol 47:32–42
Nguyen CT, Agorio A, Jossier M, Depré S, Thomine S, Filleur S (2015) Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol 57:764–775
Oh S-J, Song SI, Kim YS, Jang H-J, Kim SY, Kim M, Kim Y-K, Nahm BH, Kim J-K (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Picollo A, Pusch M (2005) Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436:420–423
Riemann M, Dhakarey R, Hazman M, Miro B, Kohli A, Nick P (2015) Exploring jasmonates in the hormonal network of drought and salinity responses. Front plant sci 6:1077
Sazegari S, Niazi A, Ahmadi FS (2015) A study on the regulatory network with promoter analysis for Arabidopsis DREB-genes. Bioinformation 11:101–106
Scheel O, Zdebik AA, Lourdel S, Jentsch TJ (2005) Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436:424
Seo JS, Joo J, Kim MJ, Kim YK, Nahm BH, Song SI, Cheong JJ, Lee JS, Kim JK, Choi YD (2011) OsbHLH148, a basic helix–loop–helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J 65:907–921
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Tampieri E, Baraldi E, Carnevali F, Frascaroli E, De Santis A (2011) The activity of plant inner membrane anion channel (PIMAC) can be performed by a chloride channel (CLC) protein in mitochondria from seedlings of maize populations divergently selected for cold tolerance. J Bioenerg Biomembr 43:611–621
Thole V, Alves SC, Worland B, Bevan MW, Vain P (2009) A protocol for efficiently retrieving and characterizing flanking sequence tags (FSTs) in Brachypodium distachyon T-DNA insertional mutants. Nat Protoc 4:650–661
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U (2007) Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J 50:466–474
von der Fecht-Bartenbach J, Bogner M, Dynowski M, Ludewig U (2010) CLC-b-mediated NO− 3/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol 51:960–968
Wang S, Su SZ, Wu Y, Li SP, Shan XH, Liu HK, Wang S, Yuan YP (2014) Overexpression of maize chloride channel gene ZmCLC-d in Arabidopsis thaliana improved its stress resistance. Biol Plant 59:55–64
Wellman CH, Gray J (2000) The microfossil record of early land plants. Philos Trans R Soc B Biol Sci 355:717–732
Wellman CH, Osterloff PL, Mohiuddin U (2003) Fragments of the earliest land plants. Nature 425:282–285
Xu Y, Buchholz WG, DeRose RT, Hall TC (1995) Characterization of a rice gene family encoding root-specific proteins. Plant Mol Biol 27:237–248
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic-and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Yang G, Zou H, Wu Y, Liu H, Yuan Y (2011) Identification and characterisation of candidate genes involved in chilling responses in maize (Zea mays L.). Plant Cell Tissue Org Cult 106:127–141
Zhang N, Si H-J, Wen G, Du H-H, Liu B-L, Wang D (2011) Enhanced drought and salinity tolerance in transgenic potato plants with a BADH gene from spinach. Plant Biotechnol Rep 5:71–77
Zifarelli G, Pusch M (2010) CLC transport proteins in plants. FEBS Lett 584:2122–2127
Acknowledgements
We heartily thank Prof. G. An for donating osclc1 mutant seeds. This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project no. PJ01121501 to Y. D. C. & PJ01364301 to G. J.) Rural Development Administration, Republic of Korea, through the National Center for GM Crops, and the National Research Foundation of Korea Grant funded by the Korean Government (MOE) [NRF-2016R1D1A1B03931167]. A graduate research assistantship to T. Y. U. and S. L. from the Brain Korea 21 Plus project of the MOE is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Um, T.Y., Lee, S., Kim, JK. et al. CHLORIDE CHANNEL 1 promotes drought tolerance in rice, leading to increased grain yield. Plant Biotechnol Rep 12, 283–293 (2018). https://doi.org/10.1007/s11816-018-0492-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0492-9