Abstract
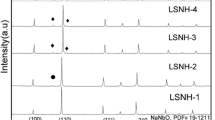
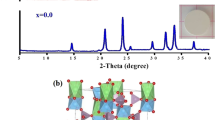
Li3/8Sr7/16−xAxZr1/4Nb3/4O3 (A = Ca, Ba) ceramics were prepared by the conventional solid-state reaction method. The structures of prepared ceramic materials changed from cubic perovskite to tetragonal tungsten bronze with increasing barium (Ba) content as indicated by the x-ray diffraction (XRD) patterns. Li3/8Sr7/16−xBaxZr1/4Nb3/4O3 (x = 0.05, 0.10) samples are mixtures of perovskite and tetragonal tungsten bronze phases. Calcium ions (Ca2+) can partially occupy the Sr sites, and thus the cubic perovskite structure remains intact. However, an unknown phase could form because of a complete substitution of Ca for Sr; the ionic conductivity measured by the alternating-current impedance spectra of samples decreases with increasing Ba and Ca content. Parent Li3/8Sr7/16Zr1/4Nb3/4O3 exhibits the highest ionic conductivity of 1.23 × 10−5 S cm−1 at 25°C. Additionally, a half cell of LiFePO4/Li performed well in charge–discharge experiments, retaining a discharge capacity of 87.9 mAhg−1 after 300 cycles when Li3/8Sr7/16Zr1/4Nb3/4O3 was selected as the solid electrolyte separator.





Similar content being viewed by others
References
E. Quartarone and P. Mustarelli, Chem. Soc. Rev. 40, 2525 (2011).
K. Takada, Acta Mater. 61 (3), 759 (2013).
N. Ohta, K. Takada, L.Q. Zhang, R.Z. Ma, M. Osada, and T. Sasaki, Adv. Mater. 18, 2226 (2006).
J. Liu and X.L. Sun, Nanotechnology 26 (2), 024001 (2015).
C.H. Chen, S. Xie, E. Sperling, A.S. Yang, G. Henriksen, and K. Amine, Solid State Ion. 167, 263 (2004).
R. Yu, Q.X. Du, B.K. Zou, Z.Y. Wen, and C.H. Chen, J. Power Sources 306, 623 (2016).
B.X. Huang, B.Y. Xu, Y.T. Li, W.D. Zhou, Y. You, S.W. Zhong, C.A. Wang, and J.B. Goodenough, ACS Appl. Mater. Int. 8, 14552 (2016).
Y.Z. Kong, Y. Li, J.Y. Lu, and C.B. Hu, J. Mater. Sci. Mater. Electr. 28, 8621 (2017).
Y.Z. Kong, Y. Li, J.W. Li, C.B. Hu, X.H. Wang, and J.Y. Lu, Ceram. Int. 44, 3947 (2017).
J.Y. Lu, Y. Li, Y.Z. Kong, and N. Zhang, Ceram. Int. 44, 4744 (2018).
J.Y. Lu and Y. Li, Electrochim. Acta 282, 409 (2018).
Y.Z. Kong, Y. Li, J.Y. Lu, X.H. Wang, and J.W. Li, Mater. Res. Express. 4, 095504 (2017).
S.D. Song, B.T. Chen, Y.L. Ruan, J. Sun, L.M. Yu, and Y. Wang, J. Thokchom. Electrochim. Acta. 270, 501 (2018).
L. Truong, J. Colter, and V. Thangadurai, Solid State Ion. 247–248, 1 (2013).
T. Venkataraman and W. Werner, J. Am. Ceram. Soc. 88, 411 (2005).
V. Thangadurai and W. Weppner, Adv. Funct. Mater. 15, 107 (2005).
K. Homma, M. Yonemura, T. Kobayashi, M. Nagao, M. Hirayama, and R. Kanno, Solid State Ion. 182, 53 (2011).
Y. Sun, W. Yan, L. An, B.B. Wu, K.F. Zhong, and R.Z. Yang, Solid State Ion. 301, 59 (2017).
S.S. Mo, P.H. Lu, F. Ding, Z.B. Xu, J.Q. Liu, X.J. Liu, and Q. Xu, Solid State Ion. 296, 37 (2016).
L. Hallopeau, D. Bregiroux, G. Rousse, D. Portehault, P. Stevens, G. Toussaint, and C. Laberty-Robert, J. Power Sources 378, 48 (2017).
Y.R. Zhao, Z. Huang, S.J. Chen, B. Chen, J. Yang, Q. Zhang, F. Ding, Y.H. Chen, and X.X. Xu, Solid State Ion. 295, 65 (2016).
C.Z. Sun, X. Huang, J. Jin, Y. Lu, Q. Wang, J.H. Yang, and Z.Y. Wen, J. Power Sources 377, 36 (2018).
K.S. Rao, P.M. Krishna, and D.M. Prasad, Phys. Stat. Sol. (b) 244 (6), 2267 (2007).
E.O. Chi, A. Gandini, K.M. Ok, L. Zhang, and P.S. Halasyamani, Chem. Mater. 16, 3616 (2004).
L.A. Bursill and B.G. Hyde, Nat. Phys. Sci. 240 (102), 122 (1972).
J. Ma, B.B. Chen, L.L. Wang, and G.L. Cui, J. Power Sources 392, 94 (2018).
Acknowledgements
The authors acknowledge the financial support provided by the National Natural Science Foundation of China (Grant Nos. 51834004, 51774076, 51704063, and 51474057). The first author is also thankful to Liaoning Key Laboratory for Metallurgical Sensor and Technology providing the facilities for the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, J., Li, Y. & Ding, Y. Structure and Conductivity of Li3/8Sr7/16−xAxZr1/4Nb3/4O3 (A = Ca, Ba) Li-ion Solid Electrolytes. JOM 72, 3256–3261 (2020). https://doi.org/10.1007/s11837-020-04239-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-020-04239-9