Abstract
Contact sensitization is the initial process involved in the development of an allergic reaction to xenobiotic environmental substances. Here, we briefly describe the differences between irritant and allergic contact dermatitis. Then, we highlight the essential steps involved in the development of an ACD reaction, i.e., the protein binding of haptens, genetic factors influencing the penetration of sensitizers into the skin, the different mechanisms driving the initial development of an inflammatory cytokine micromilieu enabling the full maturation of dendritic cells, the role of pre- and pro-haptens, antigen presentation and T cell activation via MHC and CD1 molecules, dendritic cell (DC) migration, and potential LC contribution as well as the different T cell subsets involved in ACD. In addition, we discuss the latest publications regarding factors that might influence the sensitizing potential such as repeated sensitizer application, penetration enhancers, humidity of the skin, microbiota, Tregs, and phthalates. Last but not least, we briefly touch upon novel targets for drug development that might serve as treatment options for ACD.
Similar content being viewed by others
Abbreviations
- ACD:
-
Allergic contact dermatitis
- ATP:
-
Adenosine triphosphate
- CD:
-
Contact dermatitis
- CHS:
-
Contact hypersensitivity
- DAMP:
-
Damage-associated molecular pattern
- DNCB:
-
2,4-Dinitro-1-chlorobenzene
- DNFB:
-
2,4-Dinitro-1-fluorobenzene
- DINP:
-
Di-iso-nonyl phthalate
- FITC:
-
Fluorescein isothiocyanate
- HBD:
-
Human beta defensin
- HMGB1:
-
High-mobility group box 1
- HSA:
-
Human serum albumin
- ICD:
-
Irritant contact dermatitis
- LN:
-
Lymph node
- MCI:
-
Methylchloroisothiazolinone
- OXA:
-
Oxazolone
- SDS:
-
Sodium dodecylsulfate
- PPD:
-
Para phenylenediamine
- QSAR:
-
Quantitative structure-activity relationship
- TCR:
-
T cell receptor
- TLR:
-
Toll-like receptor
- TRM :
-
(Skin) resident memory T cell
- TCM :
-
(Lymph node) resident central memory T cell
References
Martin SF. Immunological mechanisms in allergic contact dermatitis. Curr Opin Allergy Clin Immunol. 2015;15:124–30.
Willis CM (2006) Histopathology of irritant contact dermatitis. In: Irrit. Dermat. Springer, Berlin, Heidelberg, pp 345–351.
Morris-Jones R, Robertson SJ, Ross JS, White IR, McFadden JP, Rycroft RJG. Dermatitis caused by physical irritants. Br J Dermatol. 2002;147:270–5.
de Cuyper C, Lodewick E, Schreiver I, Hesse B, Seim C, Castillo-Michel H, Laux P, Luch A. Are metals involved in tattoo-related hypersensitivity reactions? A case report. Contact Dermatitis. 2017; https://doi.org/10.1111/cod.12862.
Cobb HK, Shinohara MM, Huss JT, Welch MP, Gardner JM. Systemic contact dermatitis to a surgical implant presenting as red decorative tattoo reaction. JAAD Case Rep. 2017;3:348–50.
de Groot AC. Patch testing: test concentrations and vehicles for 4350 chemicals. 3rd ed. Netherlands: Acdegroot Publishing, Wapserveen; 2008.
Diepgen TL, Ofenloch RF, Bruze M, Bertuccio P, Cazzaniga S, Coenraads P-J, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319–29.
Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J Exp Med. 1935;61:643–56.
Thierse H-J, Gamerdinger K, Junkes C, Guerreiro N, Weltzien HU. T cell receptor (TCR) interaction with haptens: metal ions as non-classical haptens. Toxicology. 2005;209:101–7.
Fitzpatrick JM, Roberts DW, Patlewicz G. What determines skin sensitization potency: myths, maybes and realities. The 500 molecular weight cut-off: an updated analysis. J Appl Toxicol. 2017;37:105–16.
Roberts DW, Mekenyan OG, Dimitrov SD, Dimitrova GD. What determines skin sensitization potency—myths, maybes and realities. Part 1. The 500 molecular weight cut-off. Contact Dermatitis. 2013;68:32–41.
Fitzpatrick JM, Roberts DW, Patlewicz G. Is skin penetration a determining factor in skin sensitization potential and potency? Refuting the notion of a LogKow threshold for skin sensitization. J Appl Toxicol. 2017;37:117–27.
Roberts DW, Aptula AO. Determinants of skin sensitisation potential. J Appl Toxicol. 2008;28:377–87.
OECD (2012) The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins Part 1: Scientific evidence. In Series on Testing and Assessment No. 168 ENV/JM/MONO(2012)10/PART1.
Kolde G, Knop J. Ultrastructural localization of 2,4-dinitrophenyl groups in mouse epidermis following skin painting with 2,4-dinitrofluorobenzene and 2,4-dinitrothiocyanatebenzene: an immunoelectron microscopical study. J Invest Dermatol. 1988;90:320–4.
Elias MS, Long HA, Newman CF, Wilson PA, West A, McGill PJ, et al. Proteomic analysis of filaggrin deficiency identifies molecular signatures characteristic of atopic eczema. J Allergy Clin Immunol. 2017; https://doi.org/10.1016/j.jaci.2017.01.039.
Thyssen JP, Linneberg A, Ross-Hansen K, Carlsen BC, Meldgaard M, Szecsi PB, et al. Filaggrin mutations are strongly associated with contact sensitization in individuals with dermatitis. Contact Dermatitis. 2013;68:273–6.
Ross-Hansen K, Linneberg A, Johansen JD, Hersoug L-G, Brasch-Andersen C, Menné T, et al. The role of glutathione S-transferase and claudin-1 gene polymorphisms in contact sensitization: a cross-sectional study. Br J Dermatol. 2013;168:762–70.
Benedetto AD, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949–63.
Bäsler K, Brandner JM. Tight junctions in skin inflammation. Pflüg Arch-Eur. J Physiol. 2017;469:3–14.
Kim D, Lee NR, Park S-Y, Jun M, Lee K, Kim S, et al. As in atopic dermatitis, nonlesional skin in allergic contact dermatitis displays abnormalities in barrier function and ceramide content. J Invest Dermatol. 2017;137:748–50.
Linauskienė K, Malinauskienė L, Blažienė A. Metals are important contact sensitizers: an experience from Lithuania. BioMed Res Int. 2017; https://doi.org/10.1155/2017/3964045.
Ahlström MG, Menné T, Thyssen JP, Johansen JD. Nickel allergy in a Danish population 25 years after the first nickel regulation. Contact Dermatitis. 2017;76:325–32.
Ahlström MG, Thyssen JP, Menné T, Johansen JD. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017; https://doi.org/10.1111/cod.12846.
Deza G, Giménez-arnau AM. Allergic contact dermatitis in preservatives: current standing and future options. Curr Opin Allergy Clin Immunol. 2017;17:263–8.
Lind M-L, Johnsson S, Lidén C, Meding B, Boman A (2017) Hairdressers’ skin exposure to hair dyes during different hair dyeing tasks. Contact Dermatitis n/a-n/a.
Parker D, Long PV, Turk JL. A comparison of the conjugation of DNTB and other dinitrobenzenes with free protein radicals and their ability to sensitize or tolerize. J Invest Dermatol. 1983;81:198–201.
Divkovic M, Pease CK, Gerberick GF, Basketter DA. Hapten–protein binding: from theory to practical application in the in vitro prediction of skin sensitization. Contact Dermatitis. 2005;53:189–200.
Jenkinson C, Jenkins RE, Aleksic M, Pirmohamed M, Naisbitt DJ, Park BK. Characterization of p-phenylenediamine–albumin binding sites and T-cell responses to hapten-modified protein. J Invest Dermatol. 2010;130:732–42.
Parkinson E, Boyd P, Aleksic M, Cubberley R, O’Connor D, Skipp P. Stable isotope labeling method for the investigation of protein haptenation by electrophilic skin sensitizers. Toxicol Sci. 2014;142:239–49.
Aleksic M, Pease CK, Basketter DA, Panico M, Morris HR, Dell A. Investigating protein haptenation mechanisms of skin sensitisers using human serum albumin as a model protein. Toxicol in Vitro. 2007;21:723–33.
Karlberg A-T, Börje A, Duus Johansen J, Lidén C, Rastogi S, Roberts D, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers – prehaptens and prohaptens. Contact Dermatitis. 2013;69:323–34.
Hagvall L, Baron JM, Börje A, Weidolf L, Merk H, Karlberg A-T. Cytochrome P450-mediated activation of the fragrance compound geraniol forms potent contact allergens. Toxicol Appl Pharmacol. 2008;233:308–13.
Natsch A, Günthardt BF, Corbi E, Pérès C, Düsterloh A, Leijs H, et al. Interlaboratory evaluation of methods to quantify skin sensitizing hydroperoxides potentially formed from linalool and limonene in perfumes. Flavour Fragr J. 2017;32:277–85.
Martin SF, Esser PR, Weber FC, Jakob T, Freudenberg MA, Schmidt M, et al. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy. 2011;66:1152–63.
Martin SF, Esser PR (2014) Chemical allergen-induced skin cell activation. In: Corsini E, Loveren H van (eds) Mol. Immunotoxicol. Wiley-VCH Verlag GmbH & Co. KGaA, pp 91–116.
Riemann H, Loser K, Beissert S, Fujita M, Schwarz A, Schwarz T, et al. IL-12 breaks dinitrothiocyanobenzene (DNTB)-mediated tolerance and converts the tolerogen DNTB into an immunogen. J Immunol. 2005;175:5866–74.
McFadden JP, Basketter DA. Contact allergy, irritancy and ‘danger’. Contact Dermatitis. 2000;42:123–7.
Piguet PF, Grau GE, Hauser C, Vassalli P. Tumor necrosis factor is a critical mediator in hapten induced irritant and contact hypersensitivity reactions. J Exp Med. 1991;173:673–9.
Friedmann PS, Sanchez-Elsner T, Schnuch A. Genetic factors in susceptibility to contact sensitivity. Contact Dermatitis. 2015;72:263–74.
Schnuch A, Westphal G, Mössner R, Uter W, Reich K. Genetic factors in contact allergy—review and future goals. Contact Dermatitis. 2011;64:2–23.
Blömeke B, Brans R, Dickel H, Bruckner T, Erdmann S, Heesen M, et al. Association between TNFA-308 G/A polymorphism and sensitization to para-phenylenediamine: a case–control study. Allergy. 2009;64:279–83.
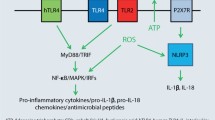
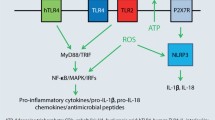
Esser PR, Wolfle U, Durr C, von Loewenich FD, Schempp CM, Freudenberg MA, et al. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS One. 2012;7:e41340.
Migdal C, Foggia L, Tailhardat M, Courtellemont P, Haftek M, Serres M. Sensitization effect of thimerosal is mediated in vitro via reactive oxygen species and calcium signaling. Toxicology. 2010;274:1–9.
El Ali Z, Gerbeix C, Hemon P, Esser PR, Martin SF, Pallardy M, et al. Allergic skin inflammation induced by chemical sensitizers is controlled by the transcription factor Nrf2. Toxicol Sci. 2013;134:39–48.
Muto J, Morioka Y, Yamasaki K, Kim M, Garcia A, Carlin AF, et al. Hyaluronan digestion controls DC migration from the skin. J Clin Invest. 2014;124:1309–19.
Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–70.
Martin SF, Dudda JC, Bachtanian E, et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205:2151–62.
Kostarnoy AV, Gancheva PG, Lepenies B, et al. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc Natl Acad Sci. 2017;114:E2758–65.
Weber FC, Esser PR, Muller T, Ganesan J, Pellegatti P, Simon MM, et al. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med. 2010;207:2609–19.
Weber FC, Németh T, Csepregi JZ, et al. Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J Exp Med. 2015;212:15–22.
Dudeck A, Dudeck J, Scholten J, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–84.
Carbone T, Nasorri F, Pennino D, Eyerich K, Foerster S, Cifaldi L, et al. CD56highCD16−CD62L−NK cells accumulate in allergic contact dermatitis and contribute to the expression of allergic responses. J Immunol. 2010;184:1102–10.
Suzuki K, Meguro K, Nakagomi D, Nakajima H. Roles of alternatively activated M2 macrophages in allergic contact dermatitis. Allergol Int. 2017;66:392–7.
Rachmawati D, Bontkes HJ, Verstege MI, Muris J, von Blomberg BME, Scheper RJ, van Hoogstraten IMW (2013) Transition metal sensing by toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermatitis 68:331–338.
Raghavan B, Martin SF, Esser PR, Goebeler M, Schmidt M. Metal allergens nickel and cobalt facilitate TLR4 homodimerization independently of MD2. EMBO Rep. 2012;13:1109–15.
Schmidt M, Raghavan B, Müller V, et al. Crucial role for human toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11:814–9.
Adam C, Wohlfarth J, Haußmann M, Sennefelder H, Rodin A, Maler M, et al. Allergy-inducing chromium compounds trigger potent innate immune stimulation via ROS-dependent Inflammasome activation. J Invest Dermatol. 2017;137:367–76.
Hartmann B, Staedtler F, Hartmann N, Meingassner J, Firat H. Gene expression profiling of skin and draining lymph nodes of rats affected with cutaneous contact hypersensitivity. Inflamm Res. 2006;55:322–34.
Kamsteeg M, Jansen PAM, Van Vlijmen-Willems IMJJ, Van Erp PEJ, Rodijk-Olthuis D, Van Der Valk PG, et al. Molecular diagnostics of psoriasis, atopic dermatitis, allergic contact dermatitis and irritant contact dermatitis. Br J Dermatol. 2010;162:568–78.
Wanke D, Mauch-Mücke K, Holler E, Hehlgans T. Human beta-defensin-2 and -3 enhance pro-inflammatory cytokine expression induced by TLR ligands via ATP-release in a P2X7R dependent manner. Immunobiology. 2016;221:1259–65.
Ferris LK, Mburu YK, Mathers AR, Fluharty ER, Larregina AT, Ferris RL, et al. Human beta-defensin 3 induces maturation of human Langerhans cell–like dendritic cells: an antimicrobial peptide that functions as an endogenous adjuvant. J Invest Dermatol. 2013;133:460–8.
Eisen HN, Kern M, Newton WT, Helmreich E. A study of the distribution of 2,4-dinitrobenzene sensitizers between isolated lymph node cells and extracellular medium in relation to induction of contact skin sensitivity. J Exp Med. 1959;110:187–206.
Agner T, Johansen JD, Overgaard L, Vølund A, Basketter D, Menné T. Combined effects of irritants and allergens. Contact Dermatitis. 2002;47:21–6.
Artik S, von Vultée C, Gleichmann E, Schwarz T, Griem P. Nickel allergy in mice: enhanced sensitization capacity of nickel at higher oxidation states. J Immunol. 1999;163:1143–52.
Watanabe H, Gehrke S, Contassot E, Roques S, Tschopp J, Friedmann PS, et al. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J Immunol. 2008;180:5826–32.
Granstein RD, Ding W, Huang J, Holzer A, Gallo RL, Di Nardo A, et al. Augmentation of cutaneous immune responses by ATP gamma S: purinergic agonists define a novel class of immunologic adjuvants. J Immunol. 2005;174:7725–31.
Shearer GM. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974;4:527–33.
Shearer GM, Rehn TG, Schmht-Verhulst A-M. Role of the murine major histocompatibility complex in the specificity of in vitro T-cell-mediated lympholysis against chemically-modified autologous lymphocytes. Immunol Rev. 1976;29:222–48.
Betts RJ, Perkovic A, Mahapatra S, Del Bufalo A, Camara K, Howell AR, et al. Contact sensitizers trigger human CD1-autoreactive T-cell responses. Eur J Immunol. 2017;47:1171–80.
Kim JH, Hu Y, Yongqing T, et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat Immunol. 2016;17:1159–66.
Wakabayashi T, D-L H, Tagawa Y-I, Sekikawa K, Iwakura Y, Hanada K, et al. IFN-γ and TNF-α are involved in urushiol-induced contact hypersensitivity in mice. Immunol Cell Biol. 2005;83:18–24.
Sawada Y, Honda T, Hanakawa S, et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J Exp Med. 2015;212:1921–30.
Lv J, Zou L, Zhao L, Yang W, Xiong Y, Li B, et al. Leukotriene B4—leukotriene B4 receptor axis promotes oxazolone-induced contact dermatitis by directing skin homing of neutrophils and CD8+ T cells. Immunology. 2015;146:50–8.
Robb CT, McSorley HJ, Lee J, et al. Prostaglandin E2 stimulates adaptive IL-22 production and promotes allergic contact dermatitis. J Allergy Clin Immunol. 2017; https://doi.org/10.1016/j.jaci.2017.04.045.
Ufer F, Vargas P, Engler JB, et al. Arc/Arg3.1 governs inflammatory dendritic cell migration from the skin and thereby controls T cell activation. Sci Immunol. 2016;1:eaaf8665–5.
Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–20.
Igyarto BZ, Jenison MC, Dudda JC, Roers A, Müller W, Koni PA, et al. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and Langerhans cell-derived IL-10. J Immunol. 2009;183:5085–93.
Bennett CL, Rijn E, van Jung S, Inaba K, Steinman RM, Kapsenberg ML, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–76.
Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56.
Honda T, Nakajima S, Egawa G, Ogasawara K, Malissen B, Miyachi Y, et al. Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol. 2010;125:1154–1156.e2.
Agüero MG, de Vocanson M, Hacini-Rachinel F, Taillardet M, Sparwasser T, Kissenpfennig A, et al. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8+ T cells and activating Foxp3+ regulatory T cells. J Clin Invest. 2012;122:1700–11.
Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–8.
Martin SF, Dudda JC, Delattre V, Bachtanian E, Leicht C, Burger B, et al. Fas-mediated inhibition of CD4+ T cell priming results in dominance of type 1 CD8+ T cells in the immune response to the contact sensitizer trinitrophenyl. J Immunol. 2004;173:3178–85.
Vocanson M, Hennino A, Rozieres A, Cluzel-Tailhardat M, Poyet G, Valeyrie M, et al. Skin exposure to weak and moderate contact allergens induces IFN[gamma] production by lymph node cells of CD4+ T-cell-depleted mice. J Invest Dermatol. 2008;129:1185–91.
Martin S, Lappin MB, Kohler J, Delattre V, Leicht C, Preckel T, et al. Peptide immunization indicates that CD8+ T cells are the dominant effector cells in trinitrophenyl-specific contact hypersensitivity. J Invest Dermatol. 2000;115:260–6.
Cavani A, Pennino D, Eyerich K. Th17 and Th22 in skin allergy. In: Ring J, Darsow U, Behrendt H, editors. Chem. Immunol. Allergy. Basel: KARGER; 2012. p. 39–44.
Cavani A, Albanesi C, Traidl C, Sebastiani S, Girolomoni G. Effector and regulatory T cells in allergic contact dermatitis. Trends Immunol. 2001;22:118–20.
Saint-Mezard P, Berard F, Dubois B, Kaiserlian D, Nicolas JF. The role of CD4+ and CD8+ T cells in contact hypersensitivity and allergic contact dermatitis. Eur J Dermatol. 2004;14:131–8.
Purath U, Ibrahim R, Zeitvogel J, Renz H, Runkel F, Schmidts T, et al. Efficacy of T-cell transcription factor–specific DNAzymes in murine skin inflammation models. J Allergy Clin Immunol. 2016;137:644–647.e8.
Jiang X, Park CO, Sweeney JG, Yoo MJ, Gaide O, Kupper TS. Dermal γδ T cells do not freely re-circulate out of skin and produce IL-17 to promote neutrophil infiltration during primary contact hypersensitivity. PLoS One. 2017;12:e0169397.
Bechara R, Antonios D, Azouri H, Pallardy M. Nickel sulfate promotes IL-17A producing CD4+ T-cells by an IL-23 dependent mechanism regulated by TLR4 and Jak-STAT pathways. J Invest Dermatol. 2017; https://doi.org/10.1016/j.jid.2017.05.025.
Bourayne M, de Gallais Y, Ali ZE, Rousseau P, Damiens M-H, Cochet C, et al. Protein kinase CK2 controls T-cell polarization through dendritic cell activation in response to contact sensitizers. J Leukoc Biol. 2017;101:703–15.
Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. 2015;21:647–53.
Schmidt JD, Ahlström MG, Johansen JD, et al. Rapid allergen-induced interleukin-17 and interferon-γ secretion by skin-resident memory CD8+ T cells. Contact Dermatitis. 2017;76:218–27.
Lidén C, Yazar K, Johansen JD, Karlberg A-T, Uter W, White IR. Comparative sensitizing potencies of fragrances, preservatives, and hair dyes. Contact Dermatitis. 2016;75:265–75.
Oakes T, Popple AL, Williams J, et al. The T cell response to the contact sensitizer paraphenylenediamine is characterized by a polyclonal diverse repertoire of antigen-specific receptors. Front Immunol. 2017; https://doi.org/10.3389/fimmu.2017.00162.
Esser PR, Kimber I, Martin SF. Correlation of contact sensitizer potency with T cell frequency and TCR repertoire diversity. EXS. 2014;104:101–14.
Paramasivan P, Lai C, Pickard C, Ardern-Jones M, Healy E, Friedmann PS. Repeated low-dose skin exposure is an effective sensitizing stimulus, a factor to be taken into account in predicting sensitization risk. Br J Dermatol. 2010;162:594–7.
van Och FMM, Vandebriel RJ, De Jong WH, van Loveren H. Effect of prolonged exposure to low antigen concentration for sensitization. Toxicology. 2003;184:23–30.
White JML, Basketter DA, Pease CK, Sanders DA, McFadden JP. Intermittent exposure to low-concentration paraphenylenediamine can be equivalent to single, higher-dose exposure. Contact Dermatitis. 2007;56:262–5.
Bonefeld CM, Nielsen MM, Rubin IMC, Vennegaard MT, Dabelsteen S, Gimenéz-Arnau E, et al. Enhanced sensitization and elicitation responses caused by mixtures of common fragrance allergens. Contact Dermatitis. 2011;65:336–42.
Bonefeld CM, Geisler C, Gimenéz-Arnau E, Lepoittevin J-P, Uter W, Johansen JD. Immunological, chemical and clinical aspects of exposure to mixtures of contact allergens. Contact Dermatitis. 2017;77:133–42.
Kienhuis AS, Slob W, Gremmer ER, Vermeulen JP, Ezendam J. A dose-response modeling approach shows that effects from mixture exposure to the skin sensitizers isoeugenol and cinnamal are in line with dose addition and not with synergism. Toxicol Sci Off J Soc Toxicol. 2015; https://doi.org/10.1093/toxsci/kfv109.
Allenby CF, Basketter DA. Minimum eliciting patch test concentrations of cobalt. Contact Dermatitis. 1989;20:185–90.
Bonefeld CM, Nielsen MM, Vennegaard MT, Johansen JD, Geisler C, Thyssen JP. Nickel acts as an adjuvant during cobalt sensitization. Exp Dermatol. 2015;24:229–31.
McLelland J, Shuster S, Matthews JNS. ‘Irritants’ increase the response to an allergen in allergic contact dermatitis. Arch Dermatol. 1991;127:1016–9.
Martin SF. Adaptation in the innate immune system and heterologous innate immunity. Cell Mol Life Sci CMLS. 2014;71:4115–30.
Martin SF. Mechanistic understanding of contact allergy. Cosmetics. 2016;3:8.
Doi T, Mizukawa Y, Shimoda Y, Yamazaki Y, Shiohara T. Importance of water content of the stratum corneum in mouse models for contact hypersensitivity. J Invest Dermatol. 2017;137:151–8.
Hacini-Rachinel F, Agüero MG, de Kanjarawi R, et al. Intestinal dendritic cell licensing through TLR4 is required for oral tolerance in allergic contact dermatitis. J Allergy Clin Immunol. 2017; https://doi.org/10.1016/j.jaci.2017.02.022.
Yasuike R, Tamagawa-Mineoka R, Ueta M, Nakamura N, Kinoshita S, Katoh N. The role of toll-like receptor 3 in chronic contact hypersensitivity induced by repeated elicitation. J Dermatol Sci. 2017; https://doi.org/10.1016/j.jdermsci.2017.07.017.
Mahnke K, Useliene J, Ring S, Kage P, Jendrossek V, Robson SC, et al. Down-regulation of CD62L shedding in T cells by CD39+ regulatory T cells leads to defective sensitization in contact hypersensitivity reactions. J Invest Dermatol. 2017;137:106–14.
Neuberger A, Ring S, Silva-Vilches C, Schrader J, Enk A, Mahnke K. Expression of CD73 slows down migration of skin dendritic cells, affecting the sensitization phase of contact hypersensitivity reactions in mice. J Dermatol Sci. 2017; https://doi.org/10.1016/j.jdermsci.2017.07.002.
Kimber I, Dearman RJ. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology. 2010;271:73–82.
Kang J, Ding Y, Li B, Liu H, Yang X, Chen M. TRPA1 mediated aggravation of allergic contact dermatitis induced by DINP and regulated by NF-κB activation. Sci Rep. 2017;7:srep43586.
Kang J, Song J, Shen S, Li B, Yang X, Chen M. Diisononyl phthalate aggravates allergic dermatitis by activation of NF-kB. Oncotarget. 2016;7:85472–82.
Wang J, Suárez-Fariñas M, Estrada Y, Parker ML, Greenlees L, Stephens G, Krueger J, Guttman-Yassky E, Howell MD. Identification of unique proteomic signatures in allergic and non-allergic skin disease. Clin Exp Allergy 2017;47(11):1456–1467. https://doi.org/10.1111/cea.12979.
Wojciechowska M, Czajkowski R, Kowaliszyn B, Żbikowska-Gotz M, Bartuzi Z. Analysis of skin patch test results and metalloproteinase-2 levels in a patient with contact dermatitis. Adv Dermatol Allergol Dermatol Alergol. 2015;32:154–61.
Jatana S, Palmer BC, Phelan SJ, DeLouise LA. Immunomodulatory effects of nanoparticles on skin allergy. Sci Rep. 2017;7:3979.
Balmert SC, Donahue C, JR V, Erdos G, Falo LD, Little SR. In vivo induction of regulatory T cells promotes allergen tolerance and suppresses allergic contact dermatitis. J Control Release. 2017;261:223–33.
Matsubara R, Kumagai K, Shigematsu H, Kitaura K, Nakasone Y, Suzuki S, et al. Fexofenadine suppresses delayed-type hypersensitivity in the murine model of palladium allergy. Int J Mol Sci. 2017; https://doi.org/10.3390/ijms18071357.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Allergic Skin Diseases
Rights and permissions
About this article
Cite this article
Esser, P.R., Martin, S.F. Pathomechanisms of Contact Sensitization. Curr Allergy Asthma Rep 17, 83 (2017). https://doi.org/10.1007/s11882-017-0752-8
Published:
DOI: https://doi.org/10.1007/s11882-017-0752-8