Abstract
Purpose of Review
To review the clinical trial data and underlying mechanistic principles in support of the robust cardiovascular (CV) benefits, in particular, heart failure (HF) outcomes association with sodium-glucose co-transporter-2 (SGLT2) inhibitors.
Recent Findings
Several large CV outcome trials in patients with type 2 diabetes mellitus (T2DM) and with either established atherosclerotic CV disease (ASCVD) or at high risk for ASCVD reveal that SGLT2 inhibitors cause reductions in CV and HF endpoints. The reduction in ASCVD appears to be confined to those with established ASCVD on the order of ≈ 14%, as does the mortality benefit—all-cause and CV-related. However, hospitalization for HF are reduced by ≈ 33% and occur regardless of baseline patient characteristics. The unprecedented HF outcomes are theorized to occur via several possible mechanisms and include optimization of conventional ASCVD risk factors, improvement in hemodynamics, prevention of cardiac and renal remodeling, inhibition of hormone dysregulation, use of more efficient metabolic substrates, ion channel inhibition, anti-inflammatory effects, and anti-oxidant effects.
Summary
Recent evidence has unveiled the irrefutable data that SGLT2 inhibitors reduce CV events in patients with T2DM, with a profound effect on reductions in hospitalization for HF. Though several mechanisms conveying this benefit are suggested, most are based in limited data requiring further validation. Nonetheless, the arrival of SGLT2 inhibitors has ushered in a new era of CV risk reductions therapies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Greene SJ, Vaduganathan M, Khan MS, Bakris GL, Weir MR, Seltzer JH, et al. Prevalent and incident heart failure in cardiovascular outcome trials of patients with type 2 diabetes. J Am Coll Cardiol. 2018;71:1379–90.
American Diabetes Association. Standards of medical care in diabetes-2019 abridged for primary care providers. Clin Diabetes. 2019;37:11–34.
Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2019 executive summary. Endocr Pract. 2019;25:69–100.
Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on expert consensus decision pathways. Journal of the American College of Cardiology. 2018;72:3200–23.
• Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28 EMPAREG-OUTCOMES trial reporting the CV outcomes of empagliflozin.
• Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57 CANVAS trial reporting CV outcomes of canagliflozin.
• Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380, 347–357 DECLARE-TIMI58 trial reporting CV outcomes of dapagliflozin.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Petersen C. Analyse des Phloridzins. Annales Academie Science Francaise. 1835;15.
von Mering J. Ueber kunstlichen Diabetes.886. Centralbl Med Wiss. 1886;22:531.
Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9.
Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–39.
Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012;157:601–10.
Roumie CL, Min JY, D'Agostino McGowan L, Presley C, Grijalva CG, Hackstadt AJ, et al. Comparative safety of sulfonylurea and metformin monotherapy on the risk of heart failure: a cohort study. J Am Heart Assoc. 2017;6.
Packer M. Worsening heart failure during the use of DPP-4 inhibitors: pathophysiological mechanisms, clinical risks, and potential influence of concomitant antidiabetic medications. JACC Heart failure. 2018;6:445–51.
Page RL 2nd, O'Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e32–69.
Cefalu WT, Kaul S, Gerstein HC, Holman RR, Zinman B, Skyler JS, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors’ expert forum. Diabetes Care. 2018;41:14–31.
Deedwania P. Dangers of hypoglycemia in cardiac patients with diabetes. Time to Switch to Safer, Newer Drugs. J Am Coll Cardiol. 2018;72:1787–9.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J. 2018;206:11–23.
Cefalo CMA, Cinti F, Moffa S, Impronta F, Sorice GP, Mezza T, et al. Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives. Cardiovasc Diabetol. 2019;18:20.
Patorno E, Pawar A, Franklin JM et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care: a first analysis from the empagliflozin comparative effectiveness and safety (EMPRISE) study. Circulation 2019.
Patorno E, Pawar A, Schneeweiss S, et al. Real-world effectiveness of SGLT2 inhibitors vs. GLP-1 receptor agonists in patients with and without cardiovascular disease American Diabetes Association, 79th Scientific sessions. In: San Francisco; 2019.
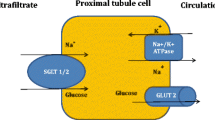
Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–97.
Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011;79:S1–6.
Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61:2079–86.
Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1845–55.
•• Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72 A comprehensive review of all systemic effects of SGLT2 inhibitors and how these integrate to ulitmately affect the heart.
Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1856–69.
•• Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018;61:2108–2117. An excellent review of heart specific effects of SGLT2 inhibitors with exceptional images.
Yanai H, Hakoshima M, Adachi H, Kawaguchi A, Waragai Y, Harigae T, et al. Effects of six kinds of sodium-glucose cotransporter 2 inhibitors on metabolic parameters, and summarized effect and its correlations with baseline data. Journal of clinical medicine research. 2017;9:605–12.
Bays HE, Sartipy P, Xu J, Sjostrom CD, Underberg JA. Dapagliflozin in patients with type II diabetes mellitus, with and without elevated triglyceride and reduced high-density lipoprotein cholesterol levels. Journal of clinical lipidology. 2017;11:450–458.e1.
Hayashi T, Fukui T, Nakanishi N, Yamamoto S, Tomoyasu M, Osamura A, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16:8.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–93.
Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138.
Heise T, Seewaldt-Becker E, Macha S, Hantel S, Pinnetti S, Seman L, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:613–21.
Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18:46.
Basu D, Huggins LA, Scerbo D, Obunike J, Mullick AE, Rothenberg PL, et al. Mechanism of increased LDL (low-density lipoprotein) and decreased triglycerides with SGLT2 (sodium-glucose cotransporter 2) inhibition. Arterioscler Thromb Vasc Biol. 2018;38:2207–16.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with Icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22.
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–58.
Karg MV, Bosch A, Kannenkeril D, Striepe K, Ott C, Schneider MP, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5.
Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, et al. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. Journal of the American Society of Nephrology : JASN. 2017;28:1867–76.
Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–87.
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62.
Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc. 2018;7.
Filippatos TD, Tsimihodimos V, Liamis G, Elisaf MS. SGLT2 inhibitors-induced electrolyte abnormalities: an analysis of the associated mechanisms. Diabetes & Metabolic Syndrome. 2018;12:59–63.
Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502.
Ndrepepa G, Braun S, King L, Hadamitzky M, Haase HU, Birkmeier KA, et al. Association of uric acid with mortality in patients with stable coronary artery disease. Metab Clin Exp. 2012;61:1780–6.
Huang H, Huang B, Li Y, Huang Y, Li J, Yao H, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16:15–24.
Verma S, Mazer CD, Yan AT. EMPA-HEART CardioLink-6 trial: a randomized trial of empagliflozin on left ventricular structure, function, and biomarkers in people with type 2 diabetes and coronary heart disease. Chicago: AHA; 2018.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310.
Li C, Zhang J, Xue M, Li X, Han F, Liu X, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18:15.
Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–6.
Buerke M, Rupprecht HJ, vom Dahl J et al. Sodium-hydrogen exchange inhibition: novel strategy to prevent myocardial injury following ischemia and reperfusion. Am J Cardiol 1999;83:19g–22g.
Theroux P, Chaitman BR, Danchin N, et al. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Guard during ischemia against necrosis (GUARDIAN) investigators. Circulation. 2000;102:3032–8.
Baartscheer A, Schumacher CA, Wust RC, et al. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60:568–73.
Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2:1025–9.
Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–71.
Labbe SM, Grenier-Larouche T, Noll C, Phoenix S, Guerin B, Turcotte EE, et al. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes. 2012;61:2701–10.
Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17:6.
Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017;16:32.
Yagi S, Hirata Y, Ise T, Kusunose K, Yamada H, Fukuda D, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetology & metabolic syndrome. 2017;9:78.
Muskiet MH, van Raalte DH, van Bommel EJ, Smits MM, Tonneijck L. Understanding EMPA-REG OUTCOME. Lancet Diabetes Endocrinol. 2015;3:928–9.
Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97.
Cherney DZ, Perkins BA, Soleymanlou N, et al. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int. 2014;86:1057–8.
Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. 2019;139:1985–7.
Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. Journal of clinical medicine research. 2016;8:844–7.
Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:356–63.
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58.
Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–7.
Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–14.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115–22.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, le Moan N, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science (New York, NY). 2013;339:211–4.
Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Phys Heart Circ Phys. 2013;304:H1060–76.
Ceriello A, Genovese S, Mannucci E, Gronda E. Glucagon and heart in type 2 diabetes: new perspectives. Cardiovasc Diabetol. 2016;15:123.
Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2011;34:1164–70.
Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc Diabetol. 2018;17:101.
Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24.
Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009;84:111–8.
Ramratnam M, Sharma RK, D'Auria S, Lee SJ, Wang D, Huang XYN, et al. Transgenic knockdown of cardiac sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2 cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1 causes pathologic hypertrophy and dysfunction in mice. J Am Heart Assoc. 2014;3.
Matsushita N, Ishida N, Ibi M, Saito M, Sanbe A, Shimojo H, et al. Chronic pressure overload induces cardiac hypertrophy and fibrosis via increases in SGLT1 and IL-18 gene expression in mice. Int Heart J. 2018;59:1123–33.
Meng L, Uzui H, Guo H, Tada H. Role of SGLT1 in high glucose level-induced MMP-2 expression in human cardiac fibroblasts. Mol Med Rep. 2018;17:6887–92.
Seidelmann SB, Feofanova E, Yu B, Franceschini N, Claggett B, Kuokkanen M, et al. Genetic variants in SGLT1, glucose tolerance, and cardiometabolic risk. J Am Coll Cardiol. 2018;72:1763–73.
Ohgaki R, Wei L, Yamada K, Hara T, Kuriyama C, Okuda S, et al. Interaction of the sodium/glucose cotransporter (SGLT) 2 inhibitor canagliflozin with SGLT1 and SGLT2. J Pharmacol Exp Ther. 2016;358:94–102.
Fitchett D. A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes Metab. 2019;21(Suppl 2):34–42.
Bersoff-Matcha SJ, Chamberlain C, Cao C, Kortepeter C, Chong WH. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med. 2019;170:764.
Kato ET, Silverman MG, Mosenzon O et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528–2536.
Furtado HM, Bonaca MP, Raz I et al. Dapagliflozin and cardiovascular outcomes in patients With type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139:2516–2527.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Cezary Wojcik and Bruce A. Warden declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Wojcik, C., Warden, B.A. Mechanisms and Evidence for Heart Failure Benefits from SGLT2 Inhibitors. Curr Cardiol Rep 21, 130 (2019). https://doi.org/10.1007/s11886-019-1219-4
Published:
DOI: https://doi.org/10.1007/s11886-019-1219-4