Abstract
Spinal cord injury causes rapid, severe osteoporosis with increased fracture risk. Mechanical unloading after paralysis results in increased osteocyte expression of sclerostin, suppressed bone formation, and indirect stimulation of bone resorption. At this time, there are no clinical guidelines to prevent bone loss after SCI, and fractures are common. More research is required to define the pathophysiology and epidemiology of SCI-induced osteoporosis. This review summarizes emerging therapeutics including anti-sclerostin antibodies, mechanical loading of the lower extremity with electrical stimulation, and mechanical stimulation via vibration therapy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2012;35:68–9.
Szollar SM, Martin EM, Sartoris DJ, et al. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998;77:28–35.
Morse LR, Battaglino RA, Stolzmann KL, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2009;20:385–92.
Morse LR, Giangregorio L, Battaglino RA, et al. VA-based survey of osteoporosis management in spinal cord injury. PMR. 2009;1:240–4.
Garland DE, Saucedo T, Reiser TV. The management of tibial fractures in acute spinal cord injury patients. Clin Orthop Relat Res. 1986;237–40.
Uebelhart D, Hartmann D, Vuagnat H, et al. Early modifications of biochemical markers of bone metabolism in spinal cord injury patients. A preliminary study. Scand J Rehabil Med. 1994;26:197–202.
Dauty M, Perrouin VB, Maugars Y, et al. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–9.
Eser P, Frotzler A, Zehnder Y, et al. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–80.
Frotzler A, Berger M, Knecht H, Eser P. Bone steady-state is established at reduced bone strength after spinal cord injury: a longitudinal study using peripheral quantitative computed tomography (pQCT). Bone. 2008;43:549–55.
Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19:48–55.
Slade JM, Bickel CS, Modlesky CM, et al. Trabecular bone is more deteriorated in spinal cord injured vs estrogen-free postmenopausal women. Osteoporos Int. 2005;16:263–72.
de Bruin ED, Dietz V, Dambacher MA, Stussi E. Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil. 2000;14:145–52.
de Bruin ED, Vanwanseele B, Dambacher MA, et al. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord. 2005;43:96–101.
Wilmet E, Ismail AA, Heilporn A, et al. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–7.
Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm, and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–5.
Frey-Rindova P, de Bruin ED, Stussi E, et al. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32.
Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15:180–9.
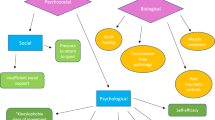
Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33.
Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–25.
Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–7.
Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–7.
Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10:537–43.
Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–9.
Staehling-Hampton K, Proll S, Paeper BW, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–52.
• Wijenayaka AR, Kogawa M, Lim HP, et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6:e25900. In this study the authors report that sclerostin up-regulates the expression of receptor activator of nuclear factor kappa B (RANKL) mRNA and down-regulates osteoprotegerin (OPG) mRNA in cultured osteoblasts, causing an increase in the RANKL:OPG mRNA ratio. These finding suggest that sclerostin exerts a catabolic effect by promoting osteoclast formation and activity in a RANKL dependent manner.
MacDonald BT, Joiner DM, Oyserman SM, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–9.
•• Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–61. The authors demonstrated that sclerostin suppressed the activity of osteoblasts in vivo. More importantly, Sost (−/−) mice were resistant to mechanical unloading-induced bone loss and to unloading-induced inhibition of bone formation. These results indicate that sclerostin is a critical mediator in the response of bone to mechanical unloading.
Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6:354.
•• Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. In this study, the authors investigated the mechanoregulation of sclerostin under increased and reduced loading conditions and found that sclerostin was dramatically reduced by loading.
Gaudio A, Pennisi P, Bratengeier C, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–53.
•• Battaglino RA, Sudhakar S, Lazzari A, et al. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone. 2012;51(3):600–5. In this study of 155 men with spinal cord injury, the authors demonstrate that sclerostin levels are initially increased after injury in response to mechanical unloading. This response is time-limited and as bone loss progresses in chronic SCI, circulating sclerostin is lowest in subjects with severe osteoporosis.
•• Morse LR, Sudhakar S, Danilack V, et al. Association between sclerostin and bone density in chronic spinal cord injury. the official journal of the American Society for Bone and Mineral Research. J Bone Miner Res. 2012;27:352–9. In this study of 39 subjects with chronic SCI and 10 without SCI the authors report that greater total limb bone mineral content was significantly associated with greater levels of circulating clerostin. Sclerostin levels were reduced, not elevated, in subjects with SCI who use a wheelchair compared with those with SCI who walk regularly. Similarly, sclerostin levels were lower in subjects with SCI who use a wheelchair compared with persons without SCI who walk regularly. These findings suggest that circulating sclerostin is a biomarker of osteoporosis severity, not a mediator of ongoing bone loss, in long-term, chronic paraplegia.
Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–88.
Ominsky MS, Vlasseros F, Jolette J, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 25:948–59.
Tian X, Jee WS, Li X, et al. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone. 2011;48:197–201.
Padhi D, Jang G, Stouch B, et al. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26.
Bellido T. Downregulation of SOST/sclerostin by PTH: a novel mechanism of hormonal control of bone formation mediated by osteocytes. J Musculoskelet Nueronal Interact. 2006;6:358–9.
Bauman WA, Zhang RL, Morrison N, Spungen AM. Acute suppression of bone turnover with calcium infusion in persons with spinal cord injury. J Spinal Cord Med. 2009;32:398–403.
Mechanick JI, Pomerantz F, Flanagan S, et al. Parathyroid hormone suppression in spinal cord injury patients is associated with the degree of neurologic impairment and not the level of injury. Arch Phys Med Rehabil. 1997;78:692–6.
Drake MT, Srinivasan B, Modder UI, et al. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5056–62.
Yu EW, Kumbhani R, Siwila-Sackman E, Leder BZ. Acute decline in serum sclerostin in response to PTH infusion in healthy men. J Clin Endocrinol Metab. 2011;96:E1848–51.
Cheng PT, Chan C, Muller K. Cyclical treatment of osteopenic ovariectomized adult rats with PTH(1–34) and pamidronate. J Bone Miner Res. 1995;10:119–26.
Rickard DJ, Wang FL, Rodriguez-Rojas AM, et al. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39:1361–72.
Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19:61–8.
Hummel K, Craven BC, Giangregorio L. Serum 25(OH)D, PTH and correlates of suboptimal 25(OH)D levels in persons with chronic spinal cord injury. Spinal Cord. 2012. doi:10.1038/sc.2012.67.
Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73.
De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–8.
Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: results from the Framingham osteoporosis study. J Bone Miner Res. 1992;7:547–53.
Jones LM, Legge M, Goulding A. Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil. 2003;84:1068–71.
Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–8.
Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–407.
Raska Jr I, Broulik P. The impact of diabetes mellitus on skeletal health: an established phenomenon with inestablished causes? Prague Med Rep. 2005;106:137–48.
Petit MA, Beck TJ, Hughes JM, et al. Proximal femur mechanical adaptation to weight gain in late adolescence: a six-year longitudinal study. J Bone Miner Res. 2008;23:180–8.
Huang TS, Wang YH, Chen SY. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch Phys Med Rehabil. 2000;81:1582–6.
Jeon JY, Steadward RD, Wheeler GD, et al. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J Clin Endocrinol Metab. 2003;88:402–7.
Bauman WA, Spungen AM, Zhong YG, Mobbs CV. Plasma leptin is directly related to body adiposity in subjects with spinal cord injury. Horm Metab Res. 1996;28:732–6 [= Hormon- und Stoffwechselforschung = Hormones et metabolisme].
Takeda S, Karsenty G. Central control of bone formation. J Bone Miner Metab. 2001;19:195–8.
Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17.
Enjuanes A, Supervia A, Nogues X, Diez-Perez A. Leptin receptor (OB-R) gene expression in human primary osteoblasts: confirmation. J Bone Miner Res. 2002;17:1135. author reply 1136.
Thomas T. Leptin: a potential mediator for protective effects of fat mass on bone tissue. Joint Bone Spine. 2003;70:18–21 [revue du rhumatisme].
Berner HS, Lyngstadaas SP, Spahr A, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–9.
No authors listed. Adiponectin is a metabolic link between obesity and bone mineral density. Exp Biol Med. 2008;233:12.
Basurto L, Galvan R, Cordova N, et al. Adiponectin is associated with low bone mineral density in elderly men. Eur J Endocrinol. 2009;160:289–93.
Gonnelli S, Caffarelli C, Del Santo K, et al. The relationship of ghrelin and adiponectin with bone mineral density and bone turnover markers in elderly men. Calcif Tissue Int. 2008;83:55–60.
Jurimae J, Jurimae T, Ring-Dimitriou S, et al. Plasma adiponectin and insulin sensitivity in overweight and normal-weight middle-aged premenopausal women. Metab Clin Exp. 2009;58:638–43.
Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92:1385–90.
de Brito CM M, Battistella LR, Saito ET, Sakamoto H. Effect of alendronate on bone mineral density in spinal cord injury patients: a pilot study. Spinal Cord. 2005;43:341–8.
Zehnder Y, Risi S, Michel D, et al. Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res. 2004;19:1067–74.
Chow JW, Jagger CJ, Chambers TJ. Characterization of osteogenic response to mechanical stimulation in cancellous bone of rat caudal vertebrae. Am J Physiol. 1993;265:E340–7.
Hsieh YF, Robling AG, Ambrosius WT, et al. Mechanical loading of diaphyseal bone in vivo: the strain threshold for an osteogenic response varies with location. J Bone Miner Res. 2001;16:2291–7.
Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204:3389–99.
Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–54.
Belanger M, Stein RB, Wheeler GD, et al. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81:1090–8.
Hagiwara T, Bell WH. Effect of electrical stimulation on mandibular distraction osteogenesis. J Craniomaxillofac Surg. 2000;28:12–9.
Zerath E, Canon F, Guezennec CY, et al. Electrical stimulation of leg muscles increases tibial trabecular bone formation in unloaded rats. J Appl Physiol. 1995;79:1889–94.
Arija-Blazquez A, Ceruelo-Abajo S, Diaz-Merino MS, et al. Time-course response in serum markers of bone turnover to a single-bout of electrical stimulation in patients with recent spinal cord injury. Eur J Appl Physiol. 2012. doi:10.1007/s00421-012-2416-7.
Dudley-Javoroski S, Saha PK, Liang G, et al. High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos Int. 2011;12:1–12.
Groah SL, Lichy AM, Libin AV, Ljungberg I. Intensive electrical stimulation attenuates femoral bone loss in acute spinal cord injury. PMR. 2010;2:1080–7.
Frotzler A, Coupaud S, Perret C, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43:169–76.
Dolbow DR, Gorgey AS, Cifu DX, et al. Feasibility of home-based functional electrical stimulation cycling: case report. Spinal Cord. 2012;50:170–1.
Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61.
Davis R, Sanborn C, Nichols D, et al. The effects of whole body vibration on bone mineral density for a person with a spinal cord injury: a case study. APAQ. 2010;27:60–72.
Asselin P, Spungen AM, Muir JW, et al. Transmission of low-intensity vibration through the axial skeleton of persons with spinal cord injury as a potential intervention for preservation of bone quantity and quality. J Spinal Cord Med. 2011;34:52–9.
Tsuchida K, Nakatani M, Hitachi K, et al. Activin signaling as an emerging target for therapeutic interventions. CCS. 2009;7:15.
Boonen S, Rosenberg E, Claessens F, et al. Inhibition of cathepsin K for treatment of osteoporosis. Curr Osteoporos Rep. 2012;10:73–9.
Disclosure
The authors reported no potential conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Battaglino, R.A., Lazzari, A.A., Garshick, E. et al. Spinal Cord Injury-Induced Osteoporosis: Pathogenesis and Emerging Therapies. Curr Osteoporos Rep 10, 278–285 (2012). https://doi.org/10.1007/s11914-012-0117-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-012-0117-0