Abstract
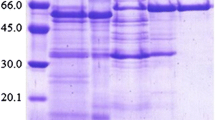
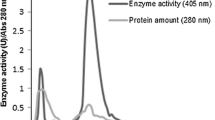
The objective of this study was to investigate a new protein with α-glucosidase inhibitory activity from the rhizomes of Zingiber ottensii. With a simple salting-out technique followed by single-step anion-exchange purification, the protein was successfully purified from the rhizomes. This protein was found to have three likely sub-unit types, 32.5, 15.2, and 13.8 kDa, as revealed by native and reducing SDS-PAGE analysis. Determination of the kinetics of the inhibition of α-glucosidase from Saccharomyces cerevisiae by standard enzymatic methods indicated the maximum percent inhibition; IC50 and K i of this protein were 77.5%, 30.15 μg/ml, and 140 μmol, while the K m and V max were 2.35 μmol and 0.11 mM/min, respectively. The inhibitory action was pH-independent within the pH range 2–10, but was potentially affected by buffer salts, and was relatively temperature-stable between 4–35 °C, with a maximum activity at 65 °C. The amino acid sequence of an internal fragment of this purified Z. ottensii rhizomal protein had a similarity to the sequence from the plant cysteine proteinase family. Although this α-glucosidase inhibitory protein was purified from Z. ottensii rhizomes and preliminarily characterized, further studies are needed prior to firm applications being envisaged.








Similar content being viewed by others
References
Park, H., Hwang, K. Y., Kim, Y. H., Oh, K. H., Lee, J. Y., & Kim, K. (2008). Bioorganic & Medicinal Chemistry Letters, 18, 3711–3715.
Gao, H., & Kawabata, J. (2005). Bioorganic & Medicinal Chemistry, 13, 1661–1671.
Zhu, Y. P., Yin, L. J., Cheng, Y. Q., Yamaki, K., Mori, Y., Su, Y. C., et al. (2008). Food Chemistry, 109, 737–742.
Taylor, J. R. N. (1994). Journal of the Institute of Brewing, 100, 417–419.
De Melo, E. B., Gomes, A. D., & Carvalho, I. (2006). Tetrahedron, 62, 10277–10302.
Whitby, K., Pierson, T., Geiss, B., Lane, K., Engle, M., Zhou, Y., et al. (2005). Journal of Virology, 79, 8698–8706.
Iwata, H., Suzuki, T., Takahashi, K., & Aramaki, I. (2002). Journal of Bioscience and Bioengineering, 93, 296–302.
Malá, Š., Karasová, P., Marková, M., & Králová, B. (2001). Czech Journal of Food Sciences, 19, 57–61.
Ladas, S., Frydas, A., Papadopoulos, A., & Raptis, S. (1992). Gut, 33, 1246–1248.
Evan, S. V., Gatehouse, A. M. R., & Fellows, L. E. (1985). Entomologia Experimentalis et Applicata, 37, 257–261.
Lee, D. (2000). Journal of Bioscience and Bioengineering, 89, 271–273.
Lee, D., & Lee, S. (2001). FEBS Letter, 501, 84–86.
Jong-Anurakkun, N., Bhandari, M. R., & Kawabata, J. (2007). Food Chemistry, 103, 1319–1323.
Yogisha, S., & Raveesha, K. A. (2009). Pharmacologyonline, 1, 404–409.
Sancheti, S., Sancheti, S., & Seo, S. U. (2009). American Journal of Pharmacology and Toxicology, 4, 8–11.
Tunsaringkarn, T., Rungsiyothin, A., & Rungrungsi, N. (2009). The Public Health Journal of Burapha University, 4, 54–63.
Van de Laar, F., Lucassen, P., Akkermans, R., Van de Lisdonk, E., Rutten, G., & Van Weel. (2005). Diabetes Care, 28, 166–175.
Tsujimoto, T., Shioyama, E., Moriya, K., Kawaratani, H., Shirai, Y., Toyohara, M., et al. (2008). World Journal of Gastroenterology, 14, 6087–6092.
Ravindran, P., & Babu, K. (2005). In ginger: The genus Zingiber. USA: CRC Press.
Akiyama, K., Kikuzaki, H., Aoki, T., Okuda, A., Lajis, N., & Nakatani, N. (2006). Journal of Natural Products, 69, 1637–1640.
Boukouvalas, J., & Wang, J. (2008). Organic Letters, 10, 3397–3399.
Tiptara, P., Petsom, A., Roengsumran, S., & Sangvanich, P. (2008). Journal of the Science of Food and Agriculture, 88, 1025–1034.
Samarkina, O., Popova, A., Gvozdik, E., Chkalina, V., Zvyagin, I., Rylova, Y., et al. (2009). Protein Expression and Purification, 65, 108–113.
Tipthara, P., Sangvanich, P., Macth, M., & Petsom, A. (2007). Journal of Plant Biology, 50, 167–173.
Bollag, D. M., Rozycki, M. D., & Edelstein, S. J. (1996). In protein methods (2nd ed.). Wiley-Liss, Inc: New York.
Lamelli, U. K. (1970). Nature, 227, 680–685.
Mortz, E., Vorm, O., Mann, M., & Roepstorff, P. (1994). Biological Mass Spectrometry, 23, 249–261.
Wang, H., & Ng, T. (2003). Protein Expression and Purification, 28, 9–14.
Lee, H. (2005). Journal of Agricultural and Food Chemistry, 53, 2446–2450.
Boonmee, A., Reynolds, C., & Sangvanich, P. (2007). Planta Medica, 73, 1197–1201.
Ahmed, K. S. O. H., Milosavić, N. B., Popović, M. M., Prodanović, R. M., Knežević, Z. D., & Jankov, R. M. (2007). Journal of the Serbian Chemical Society, 72, 1255–1263.
Agrawal, P. B., & Pandit, A. B. (2003). Biochemical Engineering Journal, 15, 37–45.
Shevchenko, A., Sunyaev, S., Loboda, A., Shevchenko, A., Bork, P., Ens, W., et al. (2001). Analytical Chemistry, 73, 1917–1926.
Acknowledgements
The authors thank the 90th Anniversary of Chulalongkorn University fund for financial support of this research. The Institute of Biotechnology and Genetic Engineering and Biotechnology program, the Faculty of Science, Chulalongkorn University, are both acknowledged for support and facilities. We also thank Dr. Robert Butcher (Publication Counselling Unit, Chulalongkorn University) for his constructive comments in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiengburanatam, N., Boonmee, A., Sangvanich, P. et al. A Novel α-Glucosidase Inhibitor Protein from the Rhizomes of Zingiber ottensii Valeton. Appl Biochem Biotechnol 162, 1938–1951 (2010). https://doi.org/10.1007/s12010-010-8971-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-8971-7