Abstract
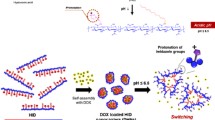
Hyaluronic acid is a naturally ionic polysaccharide with cancer cell selectivity. It is an ideal candidate material for delivery of anticancer agents. In this study, hyaluronic acid (HA) micro-hydrogel loaded with anticancer drugs was prepared by the biotin–avidin system approach. Firstly, carboxyl groups on HA were changed into amino groups with adipic acid dihydrazide (ADH) to graft with biotin by 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride named as HA–biotin. When HA–biotin solution mixed with doxorubicin hydrochloride (DOX·HCl) was blended with neutravidin, the micro-hydrogels would be formed with DOX loading. If excess biotin was added into the microgel, it would be disjointed, and DOX will be released quickly. The results of the synthesis procedure were characterized by 1H-NMR and FTIR; ADH and biotin have been demonstrated to graft on the HA molecule. A field emission scanning electron microscope was used to observe morphologies of HA micro-hydrogels. Furthermore, the in vitro DOX release results revealed that the release behaviors can be adjusted by adding biotin. Therefore, the HA micro-hydrogel can deliver anticancer drugs efficiently, and the rate of release can be controlled by biotin-specific bonding with the neutravidin. Consequently, the micro-hydrogel will perform the promising property of switching in the specific site in cancer therapy.








Similar content being viewed by others
References
Bae, K. H., Yoon, J. J., & Park, T. G. (2006). Fabrication of hyaluronic acid hydrogel beads for cell encapsulation. Biotechnology Progress, 22, 297–302.
Jeon, O., Song, S. J., Lee, K. J., Park, M. H., Lee, S. H., Hahn, S. K., et al. (2007). Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydrate Polymers, 70, 251–257.
Luo, Y., Ziebell, M. R., & Prestwich, G. D. (2000). A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules, 1, 208–218.
Yadav, A. K., Mishra, P., & Agrawal, G. P. (2008). An insight on hyaluronic acid in drug targeting and drug delivery. Journal of Drug Targeting, 16, 91–107.
Ossipov, D. A. (2010). Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert Opinion on Drug Delivery, 7, 681–703.
Lee, H., Ahn, C. H., & Park, T. G. (2009). Poly [lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer micelle nanoparticles for target-specific delivery of doxorubicin. Macromolecular Bioscience, 9, 336–342.
Park, W., Bae, B., Kim, Y. H., & Na, K. (2010). Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. European Journal of Pharmaceutical Sciences, 40, 367–375.
Cho, H. J., Yoon, I. S., Yoon, H. Y., Koo, H., Jin, Y. J., Ko, S. H., et al. (2012). Polyethylene glycol-conjugated hyaluronic acid-ceramide self-assembled nanoparticles for targeted delivery of doxorubicin. Biomaterials, 33, 1190–1200.
Yadav, A. K., Mishra, P., Mishra, A. K., Jain, S., & Agrawal, G. P. (2007). Development and characterization of hyaluronic acid-anchored PLGA nanoparticulate carriers of doxorubicin. Nanomedicine: Nanotechnology, Biology and Medicine, 3, 246–257.
Vercruysse, K. P., Marecak, D. M., Marecek, J. F., & Prestwich, G. D. (1997). Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid. Bioconjugate Chemistry, 8, 686–694.
Luo, Y., & Prestwich, G. D. (1999). Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjugate Chemistry, 10, 755–763.
Prestwich, G. D., Marecak, D. M., Marecek, J. F., Vercruysse, K. P., & Ziebell, M. R. (1998). Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives. Journal of Controlled Release, 53, 93–103.
Xiong, M. P., Forrest, M. L., Angela, L., & Kwon, G. S. (2007). Biotin-triggered release of poly (ethylene glycol)-avidin from biotinylated polyethylenimine enhances in vitro gene expression. Bioconjugate Chemistry, 18, 746–753.
Liu, Y., Liu, J., Xu, J., Feng, S., & Davis, T. P. (2010). Biodegradable PEG hydrogels cross-linked using biotin-avidin interactions. Australian Journal of Chemistry, 63, 1413–1417.
MacKinnon, N., Guérin, G., Liu, B., Gradinaru, C. C., & Macdonald, P. M. (2009). Liposome–hydrogel bead complexes prepared via biotin–avidin conjugation. Langmuir, 25, 9413–9423.
Kim, J., Nayak, S., & Lyon, L. A. (2005). Bioresponsive hydrogel microlenses. Journal of the American Chemical Society, 127, 9588–9592.
Kim, J., Singh, N., & Lyon, L. A. (2007). Influence of ancillary binding and nonspecific adsorption on bioresponsive hydrogel microlenses. Biomacromolecules, 8, 1157–1161.
Clapper, J. D., Pearce, M. E., Guymon, C. A., & Salem, A. K. (2008). Biotinylated biodegradable nanotemplated hydrogel networks for cell interactive applications. Biomacromolecules, 9, 1188–1194.
Segura, T., Anderson, B. C., Chung, P. H., Webber, R. E., Shull, K. R., & Shea, L. D. (2005). Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials, 26, 359–371.
Jia, X., Yeo, Y., Clifton, R. J., Jiao, T., Kohane, D. S., Kobler, J. B., et al. (2006). Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules, 7, 3336–3344.
Picotti, F., Fabbian, M., Gianni, R., Sechi, A., Stucchi, L., Bosco, M. (2012) Hyaluronic acid lipoate: synthesis and physicochemical properties. Carbohydrate polymers (in press).
Su, W. Y., Chen, Y. C., & Lin, F. H. (2010). Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomaterialia, 6, 3044–3055.
Gewirtz, D. A. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology, 57, 727–741.
Sun, H., Guo, B., Li, X., Cheng, R., Meng, F., Liu, H., et al. (2010). Shell-sheddable micelles based on dextran-SS-poly (ε-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules, 11, 848–854.
Speth, P., Van Hoesel, Q., & Haanen, C. (1988). Clinical pharmacokinetics of doxorubicin. Clinical Pharmacokinetics, 15, 15.
Bosio, V. E., Machain, V., López, A. G., De Berti, I. O. P., Marchetti, S. G., Mechetti, M., et al. (2012). Binding and encapsulation of doxorubicin on smart pectin hydrogels for oral delivery. Applied Biochemistry and Biotechnology, 167, 1365–1376.
Acknowledgments
The authors are thankful to the National Natural Science Foundation of China (50903009), Jilin science & technology department, science and technology development project (20100115, 20070566) and Science & Technology Bureau of Changchun City project (08SF58) for financial support to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Y., Li, Y., Duan, Q. et al. Preparation of Hyaluronic Acid Micro-Hydrogel by Biotin–Avidin-Specific Bonding for Doxorubicin-Targeted Delivery. Appl Biochem Biotechnol 169, 239–249 (2013). https://doi.org/10.1007/s12010-012-9968-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9968-1