Abstract
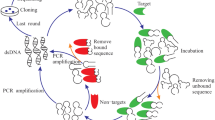
Tuberculosis (TB) remains to be a major global health problem, with about 9 million new cases and 1.4 million deaths in 2011. For the control of tuberculosis as well as other infectious diseases, WHO recommended “ASSURED” (Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, and Deliverable to the end user) diagnostic tools that can easily be maintained and used in developing countries. Aptamers are promising tools for developing point-of-care diagnostic assays for TB. In this study, ssDNA aptamers that recognize Mycobacterium tuberculosis H37Ra were selected by systematic evolution of ligands by exponential enrichment (SELEX). For this purpose, two different selection protocols, ultrafiltration and centrifugation, were applied. A total of 21 TB specific aptamers were selected. These aptamers exhibited “G-rich” regions on the 3′ terminus of the aptamers, including a motif of “TGGGG,” “GTGG,” or “CTGG.” Binding capability of selected aptamers were investigated by quantitative PCR and Mtb36 DNA aptamer was found the most specific aptamer to M. tuberculosis H37Ra. The dissociation constant (K d) of Mtb36 aptamer was calculated as 5.09 ± 1.43 nM in 95 % confidence interval. Relative binding ratio of Mtb36 aptamer to M. tuberculosis H37Ra over Mycobacterium bovis and Escherichia coli was also determined about 4 times and 70 times more, respectively. Mtb36 aptamer is highly selective for M. tuberculosis, and it can be used in an aptamer-based biosensor for the detection of M. tuberculosis.







Similar content being viewed by others
References
Njiru, Z. K. (2012). Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Neglected Tropical Diseases, 6(6), e1572.
Tiemersma, E. W., Van der Werf, M. J., Borgdorff, M. W., Williams, B. G., & Nagelkerke, N. J. (2011). Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV-negative patients: a systematic review. PLoS One, 6(4), e17601.
Dheda, K., Ruhwald, M., Theron, G., Peter, J., & Yam, W. C. (2013). Point-of-care diagnosis of tuberculosis: past, present and future. Respirology, 18, 217–232.
Rotherham, L. S., Maserumule, C., Dheda, K., Theron, J., & Khati, M. (2012). Selection and application of ssDNA aptamers to detect active TB from sputum samples. PLoS One, 7(10), e46862.
Steingart, K. R., Ng, V., & Henry, M. (2006). Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. The Lancet Infectious Diseases, 6, 664–674.
Whitelaw, A., Peter, J., & Sohn, H. (2011). Comparative cost and performance of light-emitting diode microscopy in HIV-tuberculosis co-infected patients. The European Respiratory Journal, 38, 1393–1397.
Albert, H., Ademun, P. J., & Lukyamuzi, G. (2011). Feasibility of magnetic bead technology for concentration of mycobacteria in sputum prior to fluorescence microscopy. BMC Infectious Diseases, 11, 125.
Kocagöz, T., Yılmaz, E., Özkara, Ş., Kocagöz, S., Hayran, M., Sachedeva, M., & Chambers, H. F. (1993). Detection of Mycobacterium tuberculosis in sputum samples by polymerase chain reaction, using a simplified procedure. Journal of Clinical Microbiology, 31, 1435–1438.
Scott, L. E., McCarthy, K., & Gous, N. (2011). Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Medicine, 8, e1001061.
Dheda, K., Davids, V., & Lenders, L. (2010). Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One, 5, e9848.
Boehme, C. C., Nabeta, P., & Henostroza, G. (2007). Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. Journal of Clinical Microbiology, 45, 1936–1940.
Chen, F., Zhou, J., Luo, F., Mohammed, A. B., & Zhang, X. L. (2007). Aptamer from whole-bacterium SELEX as new therapeutic reagent against virulent Mycobacterium tuberculosis. Biochemical and Biophysical Research Communications, 357, 743–748.
Min, K., Cho, M., Han, S. Y., Shim, Y. B., Ku, J., & Ban, C. (2008). A simple and direct electrochemical detection of interferon-using its RNA and DNA aptamers. Biosensors and Bioelectronics, 23, 1819–1824.
Liu, Y., Kwa, T., & Revzin, A. (2012). Simultaneous detection of cell-secreted TNF-a and IFN-g using micropatterned aptamer-modified electrodes. Biomaterials, 33, 7347–7355.
Zhu, C., Liu, J., Ling, Y., Yang, H., Liu, Z., Zheng, R., Qin, L., & Hu, Z. (2012). Evaluation of the clinical value of ELISA based on MPT64 antibody aptamer for serological diagnosis of pulmonary tuberculosis. BMC Infectious Diseases, 12, 96.
Chang, C. C., Lin, S., Lee, C. H., Chuang, T. L., Hsueh, P., Lai, H. C., & Lin, C. W. (2012). Amplified surface plasmon resonance immunosensor for interferon-gamma-based on a streptavidin-incorporated aptamer. Biosensors and Bioelectronics, 37, 68–74.
Ngubane, N. A. C., Gresh, L., Pym, A., Rubin, E. J., & Khati, M. (2014). Selection of RNA aptamers against the M. tuberculosis EsxG protein using surface plasmon resonance-based SELEX. Biochemical and Biophysical Research Communications, 449, 114–119.
Tang, X. L., Zhou, Y. X., Wu, S. M., Pan, Q., Xia, B., & Zhang, X. L. (2014). CFP10 and ESAT6 aptamers as effective Mycobacterial antigen diagnostic reagents. The Journal of Infection, 69, 569–580.
Tuerk, C., & Gold, L. (1990). Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science, 249, 505–510.
Ellington, A. D., & Szostak, J. W. (1990). In vitro selection of RNA molecules that bind specific ligands. Nature, 346, 818–822.
Gold, L., Janjic, N., Jarvis, T., Schneider, D., Walker, J. J., Wilcox, S. K., & Zichi, D. (2012). Aptamers and the RNA world, past and present. Cold Spring Harbor Perspectives in Biology, 4, a003582.
Huizenga, D. E., & Szostak, J. W. (1995). A DNA aptamer that binds adenosine and ATP. Biochemistry, 34, 656–665.
Qin, L., Zheng, R., Ma, Z., Feng, Y., Liu, Z., Yang, H., Wang, J., Jin, R., Lu, J., Ding, Y., & Hu, Z. (2009). The selection and application of ssDNA aptamers against MPT64 protein in Mycobacterium tuberculosis. Clinical Chemistry and Laboratory Medicine, 47, 405–411.
Chou, S. H., Chin, K. H., & Wang, A. H. J. (2005). DNA aptamers as potential anti-HIV agents. Trends in Biochemical Sciences, 30, 231–234.
Cao, X., Li, S., Chen, L., Ding, H., Xu, H., Huang, Y., Li, J., Liu, N., Cao, W., Zhu, Y., Shen, B., & Shao, N. (2009). Combining use of a panel of ssDNA aptamers in the detection of Staphylococcus aureus. Nucleic Acids Research, 37, 4621–4628.
Sambrook, J. & Russel, D. W. (2011). Molecular Cloning 3th Ed., NY, USA.
Lianhu, Q., Zheng, R., Ma, Z., Feng, Y., Liu, Z., Yang, H., Wang, J., Jin, R., Lu, J., Ding, Y., & Hu, Z. (2009). The selection and application of ssDNA aptamers against MPT64 protein in Mycobacterium tuberculosis. Clinical Chemistry and Laboratory Medicine, 47, 405–411.
Chang, Y. C., Yang, C. Y., Sun, R. L., Cheng, Y. F., Kao, W. C., & Yang, P. C. (2013). Rapid single cell detection of Staphylococcus aureus by aptamer-conjugated gold nanoparticles. Science Reports, 3, 1863.
Mozioğlu, E., Akgöz, M., Tamerler, C., & Kocagöz, Z. T. (2014). A simple guanidinium isothiocyanate method for bacterial genomic DNA isolation. Turkish Journal of Biology, 38, 125–129.
Pinto, A., Bermudo, R. M. C., Ozalp, V. C., & O’Sullivan, C. K. (2009). Real-time apta-PCR for 20,000-fold improvement in detection limit. Molecular BioSystems, 5, 548–553.
Telenti, A., Marchesi, F., Balz, M., Bally, F., Botrger, E. C., & Bodmer, T. (1993). Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. Journal of Clinical Microbiology, 31, 175–178.
MFold Program. (2012). Available from: http://mfold.rna.albany.edu/?q=mfold. Accessed 31 Oct 2012.
Zuker, M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research, 31, 3406–3415.
SantaLucia, J. (1998). A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proceedings of the National Academy of Sciences, 95, 1460–1465.
Peyret, N. (2000). Prediction of nucleic acid hybridization: parameters and algorithms. PhD Thesis , Wayne State University, Department of Chemistry, Detroit, MI.
Bekmurzayeva, A., Sypabekova, M., & Kanayeva, D. (2013). Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberculosis, 93, 381–388.
Naidoo, N., Ramsugit, S., & Pillay, M. (2014). Mycobacterium tuberculosis pili (MTP), a putative biomarker for a tuberculosis diagnostic test. Tuberculosis, 94, 338–345.
Hamula, C. L. A., Zhang, H., Li, F., Wang, Z., Le, X. C., & Li, X. F. (2011). Selection and analytical applications of aptamers binding microbial pathogens. TrAC Trends in Analytical Chemistry, 30, 1587–1597.
Joshi, R., Janagama, H., Dwivedi, H. P., Kumar, T. M. A. S., & JaykusA, L. A. (2009). Selection, characterization, and application of DNA aptamers for the capture and detection of Salmonella enterica serovars. Molecular and Cellular Probes, 23, 20–28.
Chen, F., Zhang, X., Zhou, J., Liu, S., & Liu, J. (2012). Aptamer inhibits Mycobacterium tuberculosis (H37Rv) invasion of macrophage. Molecular Biology Reports, 39, 2157–2162.
Saberian, M., Asgari, D., Omidi, Y., Barar, J., & Hamzeiy, H. (2013). Establishment of an electrochemical RNA aptamer-based biosensor to trace nanomolar concentrations of codeine. Turkish Journal of Chemistry, 37, 366–373.
Shen, L., Chen, Z., Li, Y., Jing, P., Xie, S., He, S., He, P., & Shao, Y. (2007). A chronocoulometric aptamer sensor for adenosine monophosphate. Chemical Communications, 21, 2169–2171.
Du, Y., Li, B., Wei, H., Wang, Y., & Wang, E. (2008). Multifunctional label-free electrochemical biosensor based on an integrated aptamer. Analytical Chemistry, 80, 5110–5117.
Zuo, X., Xiao, Y., & Plaxco, K. W. (2009). High specificity, electrochemical sandwich assays based on single aptamer sequences and suitable for the direct detection of small-molecule targets in blood and other complex matrices. Journal of the American Chemical Society, 131, 6944–6945.
Fahlman, R. P., & Sen, D. (2002). DNA conformational switches as sensitive electronic sensors of analytes. Journal of the American Chemical Society, 124, 4610–4616.
Baker, B. R., Lai, R. Y., Wood, M. S., Doctor, E. H., Heeger, A. J., & Plaxco, K. W. (2006). An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. Journal of the American Chemical Society, 128, 3138–3139.
Wang, G., Zhang, J., & Murray, R. W. (2002). DNA binding of an ethidium intercalator attached to a monolayer-protected gold cluster. Analytical Chemistry, 74, 4320–4327.
Nutiu, R., & Li, Y. (2004). Structure-switching signaling aptamers: transducing molecular recognition into fluorescence signaling. Chemistry - A European Journal, 10, 1868–1876.
Nutiu, R., & Li, Y. (2003). Structure-switching signaling aptamers. Journal of the American Chemical Society, 125, 4771–4778.
Pavlov, V., Shlyahovsky, B., & Willner, I. (2005). Fluorescence detection of DNA by the catalytic activation of an aptamer/thrombin complex. Journal of the American Chemical Society, 127, 6522–6523.
Zhou, C., Jiang, Y., Hou, S., Ma, B., Fang, X., & Li, M. (2006). Detection of oncoprotein platelet-derived growth factor using a fluorescent signaling complex of an aptamer and TOTO. Analytical and Bioanalytical Chemistry, 384, 1175–1180.
Li, B., Wei, H., & Dong, S. (2007). Sensitive detection of protein by an aptamer-based label-free fluorescing molecular switch. Chemical Communications, 1, 73–75.
Swensen, J. S., Xiao, Y., Ferguson, B. S., Lubin, A. A., Lai, R. Y., Heeger, A. J., Plaxco, K. W., & Soh, H. T. (2009). Continuous, real-time monitoring of cocaine in undiluted blood serum via a microfluidic, electrochemical aptamer-based sensor. Journal of the American Chemical Society, 131, 4262–4266.
Zhou, J., Soontornworajit, B., Snipes, M. P., & Wang, Y. (2009). Development of a novel pretargeting system with bifunctional nucleic acid molecules. Biochemical and Biophysical Research Communications, 386, 521–525.
Li, T., Li, B., & Dong, S. (2007). Aptamer-based label-free method for hemin recognition and DNA assay by capillary electrophoresis with chemiluminescence detection. Analytical and Bioanalytical Chemistry, 389, 887–893.
Liu, J., & Lu, Y. (2004). Colorimetric biosensors based on DNAzyme-assembled gold nanoparticles. Journal of Fluorescence, 14, 343–354.
Elghanian, R., Storhoff, J. J., Mucic, R. C., Letsinger, R. L., & Mirkin, C. A. (1997). Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science, 277, 1078–1081.
Nahid, P., Sizemore, E. B., Jarlsberg, L. G., Grootec, M. A., Johnson, J. L., Muzanyi, G., Engle, M., Weiner, M., Janjic, N., Sterling, D. G., & Ochsner, U. A. (2014). Aptamer-based proteomic signature of intensive phase treatment response in pulmonary tuberculosis. Tuberculosis, 94, 187–196.
Chen, F., Zhou, J., Huang, Y. H., Huang, F. Y., Liu, Q., Fang, Z., Yang, S., Xiong, M., Lin, Y. Y., & Tan, G. H. (2013). Function of ssDNA aptamer and aptamer pool against Mycobacterium tuberculosis in a mouse model. Molecular Medicine Reports, 7, 669–673.
Shum, K. T., Lui, E. L., Wong, S. C., Yeung, P., Sam, L., Wang, Y., Watt, R. M., & Tanner, J. A. (2011). Aptamer-mediated inhibition of Mycobacterium tuberculosis polyphosphate kinase 2. Biochemistry, 50, 3261–3271.
Acknowledgments
This study was supported by The Scientific and Technological Research Council of Türkiye (TÜBİTAK) National Metrology Institute with project number 110S141. We would like to thank Prof. Dr. Asım Esen for his molecular biology expertise and assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mozioglu, E., Gokmen, O., Tamerler, C. et al. Selection of Nucleic Acid Aptamers Specific for Mycobacterium tuberculosis . Appl Biochem Biotechnol 178, 849–864 (2016). https://doi.org/10.1007/s12010-015-1913-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1913-7