Abstract
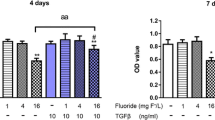
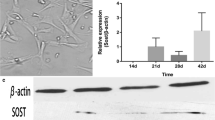
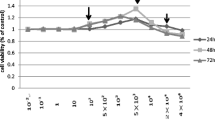
Little attention has been paid to the tolerance of osteoblasts to fluoride in distinct differentiation stages, and the role of TGF-β1 in fluoride-treated osteoblast differentiation of progenitors and precursors was rarely mentioned in previous studies. The present study aimed to clarify how fluoride affected different differentiation stages of osteoblasts, and to elucidate the role of TGF-β1 in this process. We assessed cell migration, proliferation, DNA damage, and apoptosis of early-differentiated osteoblasts derived from bone marrow stem cells (BMSCs) exposed to fluoride with or without TGF-β1. Subsequently, MC3T3-E1 cells cultured with mineral induction medium were treated with fluoride to test fluoride’s effect on late-differentiated osteoblasts. The specific fluoride concentrations and treatment times were chosen to evaluate the role of TGF-β1 in fluoride-induced osteoblastic differentiation and function. Results showed early-differentiated osteoblasts treated with a low dose of fluoride grew and moved more rapidly. TGF-β1 promoted cell proliferation and inhibited cell apoptosis in early-differentiated osteoblasts exposed to a low fluoride dose, but enhanced apoptosis at higher fluoride conditions. In the late-differentiated osteoblasts, the fluorine dose range with anabolic effects was narrowed, and the fluoride range with catabolic effects was widened. Treatment with a low fluoride dose stimulated the alkaline phosphatase (ALP) expression. TGF-β1 treatment inhibited Runx2 expression but increased RANKL expression in late-differentiated osteoblasts exposed to fluoride. Meanwhile, TGF-β1 treatments activated Smad3 phosphorylation but blocked Wnt10b expression in osteoblasts. We conclude that TGF-β1 plays an essential role in fluoride-induced differentiation and osteoblast function via activation of Smad3 instead of Wnt10 signaling.






Similar content being viewed by others
References
Nawata S, Kaneta T, Ogawa M (2017) Differences in sodium fluoride-18 uptake in the normal skeleton depending on the location and characteristics of the bone. Nuklearmedizin. 56(3):91–96. https://doi.org/10.3413/Nukmed-0867-16-12
Wang XL, Ming J, Qiu B et al (2019) Relationship between fluoride exposure, orthopedic injuries and bone formation markers in patients with coal-burning fluorosis. Ying Yong Sheng Tai Xue Bao 30(1):43–48. https://doi.org/10.13287/j.1001-9332.201901.026
Nelson EA, Halling CL, Buikstra JE (2019) Evidence of skeletal fluorosis at the Ray Site, Illinois, USA: a pathological assessment and discussion of environmental factors. Int J Paleopathol 26:48–60. https://doi.org/10.1016/j.ijpp.2019.05.003
Tamer MN, Kale Köroğlu B, Arslan C, Akdoğan M, Köroğlu M, Çam H, Yildiz M (2007) Osteosclerosis due to endemic fluorosis. Sci Total Environ 373(1):43–48. https://doi.org/10.1016/j.scitotenv.2006.10.051
Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 90(5):552–560. https://doi.org/10.1177/0022034510384626
Jiang N, Guo F, Sun B et al (2020) Different effects of fluoride exposure on the three major bone cell types. Biol Trace Elem Res 193(1):226–233. https://doi.org/10.1016/j.tox.2020.152429
Zhang J, Jiang N, Yu H, Yu X, Guo F, Zhao Z, Xu H (2019) Requirement of TGFβ signaling for effect of fluoride on osteoblastic differentiation. Biol Trace Elem Res 187(2):492–498. https://doi.org/10.1007/s12011-018-1387-x
Yu H, Jiang N, Yu X, Zhao Z, Zhang X, Xu H (2018) The role of TGFβ receptor 1-smad3 signaling in regulating the osteoclastic mode affected by fluoride. Toxicology 393:73–82. https://doi.org/10.1016/j.tox.2017.11.009
Yang C, Wang Y, Xu H (2017) Fluoride regulate osteoblastic transforming growth factor-beta 1 signaling by mediating recycling of the type I receptor ALK5. PLoS One 12(1):e0170674. https://doi.org/10.1371/journal.pone.0170674
Su P, Tian Y, Yang C, Ma X, Wang X, Pei J, Qian A (2018) Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int J Mol Sci 19(8):2343. https://doi.org/10.3390/ijms19082343
Chen G, Deng C, Li YP (2012) TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8(2):272–288. https://doi.org/10.7150/ijbs.2929
Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B, Mishina Y (2018) TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol 10(5):a022202. https://doi.org/10.1101/cshperspect.a022202
Yao Y, Ma Y, Zhong N, Pei J (2019) The inverted U-curve association of fluoride and osteoclast formation in mice. Biol Trace Elem Res 191(2):419–425. https://doi.org/10.1007/s12011-018-1624-3
Wang J, Li G, Li Y, Zhao Y, Manthari RK, Wang J (2020) The effects of fluoride on the gap-junctional intercellular communication of rats’ osteoblast. Biol Trace Elem Res 193(1):195–203. https://doi.org/10.1007/s12011-019-01692-9
Yasui T (2011) Regulation of RANKL-induced osteoclastogenesis by TGF-beta through molecular interaction between Smad3 and Traf6. J Bone Miner Res 26:1447–1456. https://doi.org/10.1002/jbmr.357
Kim KK, Ji C, Chang W et al (2006) Repetitive exposure to TGF-b suppresses TGF-b type I receptor expression by differentiated osteoblasts. Gene 379:175–184. https://doi.org/10.1016/j.gene.2006.05.005
Suzuki E, Ochiai-Shino H, Aoki H, Onodera S, Saito A, Saito A, Azuma T (2014) Akt activation is required for TGF-β1-induced osteoblast differentiation of MC3T3-E1 pre-osteoblasts. PLoS One 9(12):e112566. https://doi.org/10.1371/journal.pone.0112566
Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ (2013) TGF-β induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology 154(10):3745–3452. https://doi.org/10.1210/en.2013-1272
Morikawa M, Derynck R, Miyazono K (2016) TGF-b and the TGF-b family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8:a021873. https://doi.org/10.1101/cshperspect.a021873
Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC (2012) TGF-b regulates sclerostin expression via the ECR5 enhancer. Bone 50:663–669. https://doi.org/10.1016/j.bone.2011.11.016
Funding
This work was supported by grants from the Natural Science Foundation of Jilin Province of China (20180101151JC) and the National Natural Science Foundation of China [81673111].
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, N., Xu, W., Zhang, Z. et al. Role of TGF-β1 in Fluoride-Treated Osteoblasts at Different Stages. Biol Trace Elem Res 200, 740–748 (2022). https://doi.org/10.1007/s12011-021-02686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02686-2