Abstract
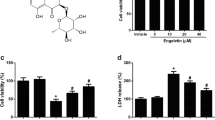
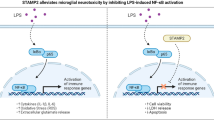
Alzheimer’s disease (AD) is a neurodegenerative disorder and the leading cause of dementia in aged populations worldwide. The deposition of toxic protein aggregates such as amyloid beta (Aβ) is a hallmark of AD, and there is growing awareness that a key driver of AD pathogenesis is the neuroinflammatory cascade triggered and sustained by these proteins. Consequently, interventions that suppress prolonged neuroinflammation represent viable therapeutic approaches for AD. In this context, we tested the natural product gedunin which is an anti-inflammatory molecule, found in the seeds of the neem tree (Azadirachta indica), whose mechanism of action remains to be fully elucidated. Using a mouse microglia cell line (IMG), we show that gedunin suppresses neuroinflammation arising from Aβ1–42 oligomer exposure. Our results demonstrate that gedunin suppresses Aβ1–42-induced NF-κB activation and its targets, including nitric oxide (NO) and IL-1β, known proinflammatory molecules. Further, we show that gedunin inhibits neuroinflammation by activating nuclear factor 2 erythroid–related factor 2 (Nrf2) and its downstream targets γ-glutamylcysteine synthetase, heme oxygenase 1, and NADPH quinone dehydrogenase 1, which are involved in quenching reactive oxygen and nitrogen species (NO) generated by NF-κB activation. Nrf2 activation appears essential for the anti-inflammatory effect because when silenced, the proinflammatory effects of Aβ1–42 are enhanced and the protective effect of gedunin against NO production is reduced. Additionally, using human neuronal cells (SH-SY5Y), we show that gedunin prevents neurotoxicity secondary to Aβ-induced microglial activation. In conclusion, our findings highlight a potential therapeutic role of gedunin in neurodegenerative diseases.






Similar content being viewed by others
References
Alzheimer’s Association (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11(3):332–384
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):270–279
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217(2):459–472
Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T (2018) Bidirectional microglia–neuron communication in health and disease. Front Cell Neurosci 12:323
Regen F, Hellmann-Regen J, Costantini E, Reale M (2017) Neuroinflammation and Alzheimer’s disease: implications for microglial activation. Curr Alzheimer Res 14(11):1140–1148
Harirforoosh S, Asghar W, Jamali F (2013) Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci 16(5):821–847
Gupta SC, Sundaram C, Reuter S, Aggarwal BB (2010) Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 1799(10–12):775–787
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661
Karimi A, Majlesi M, Rafieian-Kopaei M (2015) Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol 4(1):27–30 eCollection 2015
Alzohairy MA (2016) Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med 2016:7382506
Henriques MD, Penido C (2014) The therapeutic properties of Carapa guianensis. Curr Pharm Des 20(6):850–856
Nie S, Xu Y, Chen G, Ma K, Han C, Guo Z, Zhang Z, Ye K et al (2015) Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology 99:448–458
Borges PV, Moret KH, Raghavendra NM, Maramaldo Costa TE, Monteiro AP, Carneiro AB, Pacheco P, Temerozo JR et al (2017) Protective effect of gedunin on TLR-mediated inflammation by modulation of inflammasome activation and cytokine production: evidence of a multitarget compound. Pharmacol Res 115:65–77
Smirnova NA, Haskew-Layton RE, Basso M, Hushpulian DM, Payappilly JB, Speer RE, Ahn YH, Rakhman I et al (2011) Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem Biol 18(6):752–765
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between NF-kB response pathways. Biochem Soc Trans 43(4):621–625
Meda L, Baron P, Scarlato G (2001) Glial activation in Alzheimer’s disease: the role of Abeta and its associated proteins. Neurobiol Aging 22(6):885–893
McCarthy RC, Lu DY, Alkhateeb A, Gardeck AM, Lee CH, Wessling-Resnick M (2016) Characterization of a novel adult murine immortalized microglial cell line and its activation by amyloid-beta. J Neuroinflammation 13:21
Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Götz J (2003) Beta-amyloid induces paired helical filament-like tau filaments in tissue culture. J Biol Chem 278(41):40162–40168
Leonoudakis D, Rane A, Angeli S, Lithgow GJ, Andersen JK, Chinta SJ (2017) Anti-inflammatory and neuroprotective role of natural product securinine in activated glial cells: implications for Parkinson’s disease. Mediat Inflamm 2017:8302636
Wan F, Lenardo MJ (2010) The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res 20(1):24–33
Shih RH, Wang CY, Yang CM (2015) NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 8:77
Valerio A, Boroni F, Benarese M, Sarnico I, Ghisi V, Bresciani LG, Ferrario M, Borsani G et al (2006) NF-kappaB pathway: a target for preventing beta-amyloid (Abeta)-induced neuronal damage and Abeta42 production. Eur J Neurosci 23(7):1711–1720
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75(6):639–653
Forlenza OV, Diniz BS, Talib LL, Mendonça VA, Ojopi EB, Gattaz WF, Teixeira AL (2009) Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord 28(6):507–512
Mehlhorn G, Hollborn M, Schliebs R (2000) Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci 18(4–5):423–431
Yerra VG, Negi G, Sharma SS, Kumar A (2013) Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol 1:394–397
Vargas MR, Johnson JA (2009) The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med 11:e17
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284(20):13291–13295
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T et al (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7:11624
An YW, Jhang KA, Woo SY, Kang JL, Chong YH (2016) Sulforaphane exerts its anti-inflammatory effect against amyloid-beta peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol Aging 38:1–10
de Oliveira MR, de Souza ICC, Furstenau CR (2018) Carnosic acid induces anti-inflammatory effects in paraquat-treated SH-SY5Y cells through a mechanism involving a crosstalk between the Nrf2/HO-1 axis and NF-kappaB. Mol Neurobiol 55(1):890–897
Ahmed SM, Luo L, Namani A, Wang XJ, Tang X (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol basis Dis 1863(2):585–597
Rushworth SA, Shah S, MacEwan DJ (2011) TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol 187(2):702–707
Tseng JH, Xie L, Song S, Xie Y, Allen L, Ajit D, Hong JS, Chen X et al (2017) The deacetylase HDAC6 mediates endogenous neuritic tau pathology. Cell Rep 20(9):2169–2183
Eckert A, Marques CA, Keil U, Schüssel K, Müller WE (2003) Increased apoptotic cell death in sporadic and genetic Alzheimer’s disease. Ann N Y Acad Sci 1010:604–609
Calissano P, Matrone C, Amadoro G (2009) Apoptosis and in vitro Alzheimer disease neuronal models. Commun Integr Biol 2(2):163–169
Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S et al (2011) Aβ(1- 42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1, and GSK-3β. Nat Neurosci 14(5):545–547
Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farias GA, Maccioni RB (2014) Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. https://doi.org/10.3389/fncel.2014.00112
Casey DA, Antimisiaris D, O'Brien J (2010) Drugs for Alzheimer’s disease: are they effective? P T 35(4):208–211
Shal B, Ding W, Ali H, Kim YS, Khan S, Shal B, Ding W, Ali H et al (2018) Anti neuroinflammatory potential of natural products in attenuation of Alzheimer’s disease. Front Pharmacol 29(9):548 eCollection 2018
Funding
This work was supported by the Touro University California College of Pharmacy Master of Science in Medical Health Sciences program. MC is supported by a postdoctoral fellowship from the Larry L. Hillblom Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Tom, S., Rane, A., Katewa, A.S. et al. Gedunin Inhibits Oligomeric Aβ1–42-Induced Microglia Activation Via Modulation of Nrf2-NF-κB Signaling. Mol Neurobiol 56, 7851–7862 (2019). https://doi.org/10.1007/s12035-019-1636-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1636-9