Abstract
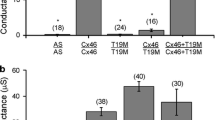
This study was designed to detect the expression, detergent resistance, subcellular localization, and channel and hemichannel functions of mutant Cx50 to understand the forming mechanism for inducing congenital cataract by a novel mutation p.S276F in connexin 50 (Cx50) reported previously by us. HeLa and human lens epithelial (HLE) cells were transfected with wild-type Cx50 and mutant Cx50 (S276F). We examined the functional characteristics of mutant Cx50 (S276F) in comparison with those of wild-type Cx50 using immunoblot, confocal fluorescence microscopy, dye transfer analysis and dye uptake assay. The mutant and wild-type Cx50 were expressed in equal levels and could efficiently localize to the plasma membrane without transportation and assembly problems. Scrape loading dye transfer was significantly evident in cells transfected with wild-type Cx50 compared to those in cells transfected with mutant Cx50 and cotransfected with wild-type and mutant Cx50. The dye uptake was found to be significantly lower in cells transfected with mutant Cx50 than in cells transfected with wild-type Cx50 and cells cotransfected with wild-type and mutant Cx50. The transfected HeLa and HLE cell lines showed similar performance in all the experiments. These results indicated that the mutant Cx50 (S276F) might inhibit the function of gap junction channel in a dominant negative manner, but inhibit the hemichannel function in a recessive negative manner.





Similar content being viewed by others
References
Abbaci M., Barberi-Heyob M., Blondel W., Guillemin F. and Didelon J. 2008 Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques 45, 33.
Arora A., Minogue P., Liu X., Addison P., Russel-Eggitt I., Webster A. et al. 2008 A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J. Med. Gen. 45, 155–160.
Banks E. A., Toloue M. M., Shi Q., Zhou Z. J., Liu J., Nicholson B. J. et al. 2009 Connexin mutation that causes dominant congenital cataracts inhibits gap junctions, but not hemichannels, in a dominant negative manner. J. Cell Sci. 122, 378–388.
Bennett M. V., Contreras J. E., Bukauskas F. F. and Sáez J. C. 2003 New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 26, 610–617.
Beyer E. C. and Berthoud V. M. 2014 Connexin hemichannels in the lens. Front. Physiol. 5, 20.
Beyer E. C., Kistler J., Paul D. L. and Goodenough D. A. 1989 Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J. Cell Biol. 108, 595–605.
D’hondt C., Srinivas S. P., Vereecke J. and Himpens B. 2007 Adenosine opposes thrombin-induced inhibition of intercellular calcium wave in corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 48, 1518–1527.
DeRosa A. M., Mui R., Srinivas M. and White T. W. 2006 Functional characterization of a naturally occurring Cx50 truncation. Invest. Ophthalmol. Vis. Sci. 47, 4474–4481.
DeRosa A. M., Meşe G., Li L., Sellitto C., Brink P. R., Gong X. et al. 2009 The cataract causing Cx50-S50P mutant inhibits Cx43 and intercellular communication in the lens epithelium. Exp. Cell Res. 315, 1063–1075.
DeVries S. and Schwartz E. 1992 Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J. Physiol. 445, 201–230.
El-Fouly M. H., Trosko J. E. and Chang C.-C. 1987 Scrape-loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 168, 422–430.
Evans W., De Vuyst E. and Leybaert L. 2006 The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem. J. 397, 1–14.
Gerido D. A. and White T. W. 2004 Connexin disorders of the ear, skin, and lens. Biochim. Biophys. Acta 1662, 159–170.
Goodenough D. A. and Paul D. L. 2003 Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 4, 285–295.
He L. Q., Liu Y., Cai F., Tan Z. P., Pan Q., Liang D. S. et al. 2005 Intracellular distribution, assembly and effect of disease-associated connexin 31 mutants in HeLa cells. Acta Biochim. Biophys. Sin. 37, 547–554.
Homma N., Alvarado J. L., Coombs W., Stergiopoulos K., Taffet S. M., Lau A. F. and Delmar M. 1998 A particle-receptor model for the insulin-induced closure of connexin43 channels. Circ. Res. 83, 27–32.
Lampe P. D. and Lau A. F. 2004 The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 36, 1171–1186.
Li J., Wang Q., Fu Q., Zhu Y., Zhai Y., Yu Y. et al. 2013 A novel connexin 50 gene (gap junction protein, alpha 8) mutation associated with congenital nuclear and zonular pulverulent cataract. Mol. Vis. 19, 767.
Li W. and Nagy J. 2000 Activation of fibres in rat sciatic nerve alters phosphorylation state of connexin-43 at astrocytic gap junctions in spinal cord: evidence for junction regulation by neuronal–glial interactions. Neurosci 97, 113–123.
Lichtenstein A., Gaietta G. M., Deerinck T. J., Crum J., Sosinsky G. E., Beyer E. C. et al. 2009 The cytoplasmic accumulations of the cataract-associated mutant, Connexin50P88S, are long-lived and form in the endoplasmic reticulum. Exp. Eye Res. 88, 600–609.
Liu J., Vitorin J. F. E., Weintraub S. T., Gu S., Shi Q., Burt J. M. et al. 2011 Phosphorylation of connexin 50 by protein kinase A enhances gap junction and hemichannel function. J. Biol. Chem. 286, 16914–16928.
Milks L. C., Kumar N. M., Houghten R., Unwin N. and Gilula N. B. 1988 Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J. 7, 2967.
Musil L. S. and Goodenough D. A. 1993 Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74, 1065–1077.
Musil L. S., Beyer E. C. and Goodenough D. A. 1990 Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J. Membr. Biol. 116, 163–175.
Ren Q, Riquelme M. A., Xu J. et al. 2013 Cataract-causing mutation of human connexin 46 impaird gap junction, but increases hemichannel function and cell death. PloS One 8, e74732.
Söhl G. and Willecke K. 2003 An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 10, 173–180.
Thomas B. C., Minogue P. J., Valiunas V., Kanaporis G., Brink P. R., Berthoud V. M. et al. 2008 Cataracts are caused by alterations of a critical N-terminal positive charge in connexin50. Invest. Ophthalmol. Vis. Sci. 49, 2549–2556.
Tong J.-J., Minogue P. J., Guo W., Chen T.-L., Beyer E. C., Berthoud V. M. et al. 2011 Different consequences of cataract-associated mutations at adjacent positions in the first extracellular boundary of connexin50. Am. J. Physiol. Cell Physiol. 300, C1055–C1064.
Valiunas V. 2002 Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 119, 147–164.
Wang L., Luo Y., Wen W., Zhang S. and Lu Y. 2011 Another evidence for a D47N mutation in GJA8 associated with autosomal dominant congenital cataract. Mol. Vis. 17, 2380.
White T. W., Bruzzone R., Goodenough D. A. and Paul D. L. 1992 Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol. Biol. Cell 3, 711.
Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Güldenagel M. et al. 2002 Structural and functional diversity of connexin genes in the mouse and human genome. J. Biol. Chem. 383, 725–737.
Xu X., Berthoud V., Beyer E. and Ebihara L. 2002 Functional role of the carboxyl terminal domain of human connexin 50 in gap junctional channels. J. Membr. Biol. 186, 101–112.
Yan M., Xiong C., Ye S. Q., Chen Y., Ke M., Zheng F. et al. 2008 A novel connexin 50 (GJA8) mutation in a Chinese family with a dominant congenital pulverulent nuclear cataract. Mol. Vis. 14, 418.
Yum S. W., Kleopa K. A., Shumas S. and Scherer S. S. 2002 Diverse trafficking abnormalities of connexin32 mutants causing CMTX. Neurobiol. Dis. 11, 43–52.
Acknowledgement
This study was supported by Health and Family Planning Commission of Hubei Province (grants JS-2011007 and QJX2010-18).
Author information
Authors and Affiliations
Corresponding author
Additional information
[Liu Y., Qiao C., Wei T., Zheng F., Guo S., Chen Q., Yan M. and Zhou X. 2015 Mutant connexin 50 (S276F) inhibits channel and hemichannel functions inducing cataract. J. Genet. 94, xx–xx]
Rights and permissions
About this article
Cite this article
LIU, Y., QIAO, C., WEI, T. et al. Mutant connexin 50 (S276F) inhibits channel and hemichannel functions inducing cataract. J Genet 94, 221–229 (2015). https://doi.org/10.1007/s12041-015-0506-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-015-0506-0