Abstract
Reports of successful transjugular intrahepatic portosystemic shunt (TIPS) surgery in patients with portal vein thrombosis (PVT) are considered anecdotal owing to the technical difficulty of the procedure and potential procedure-related complications. A literature review was undertaken to determine the feasibility and safety of TIPS in the treatment of PVT. All studies in which TIPS was attempted in patients with PVT were identified by searching through the PUBMED and MEDLINE databases. A total of 424 PVT patients undergoing TIPS were reported in 54 articles. The success rate of TIPS insertion was 67–100% in 19 case series. Further, 85 patients with portal cavernoma underwent successful TIPS insertions. Three therapeutic strategies of TIPS placement were used: (1) TIPS placement followed by portal vein recanalization via the shunt, (2) portal vein recanalization via percutaneous approaches followed by TIPS placement, and (3) TIPS insertion between a hepatic vein and a large collateral vessel without portal vein recanalization. Four approaches were used to access the portal vein: transjugular, transhepatic, transsplenic, and transmesenteric. Intra-abdominal hemorrhage secondary to hepatic capsule perforation was lethal in only three patients. No episode of pulmonary embolism was reported. Other procedure-related complications were reversible. The overall incidence of shunt dysfunction and hepatic encephalopathy was 8–33% and 0–50%, respectively. In conclusion, the reviewed studies uniformly support the feasibility and safety of TIPS for PVT even in the presence of portal cavernoma. Further, several major issues that remain unresolved are discussed.
Similar content being viewed by others
Introduction
Since the first transjugular intrahepatic portosystemic shunt (TIPS) surgery performed in a patient with continuous gastric variceal bleeding [1], the use of TIPS has progressively expanded [2]. The principal indications for TIPS include prevention of variceal rebleeding [3] and management of refractory ascites that requires repeated large-volume paracentesis [4]. On the basis of evidence from several recent case series [5, 6], the updated American Association for the Study of Liver Diseases (AASLD) practice guidelines on applications of TIPS recommend that TIPS surgery should be performed in patients with Budd–Chiari syndrome who fail to improve with anticoagulation [7]. More recently, particular attention has been paid to the early use of TIPS with covered stents as the first-line therapeutic modality in patients with acute variceal bleeding with Child-Pugh scores of class B or C [8]. However, because of the technical difficulty and potential procedure-related complications, TIPS surgery is still not widely recommended for the treatment of portal vein thrombosis (PVT) [2], and successful TIPS insertions in patients with PVT are regarded as anecdotal reports [9]. The current practice guidelines and consensus on the management of PVT recommend that anticoagulation should be used in patients with acute PVT that is unrelated to cirrhosis [9, 10], given the relatively high recanalization rate reported in previous case series [11, 12]. However, the recommendation may be challenged by two recent studies. A prospective cohort study in Europe demonstrated that recanalization occurred in only one-third of patients receiving early anticoagulation for acute PVT [13]. In another large retrospective study conducted at the Mayo Clinic, the authors concluded that anticoagulation should be minimized in PVT patients with a history of gastrointestinal variceal bleeding [14]. Taken together, these findings suggest that the role of anticoagulation in the treatment of PVT is limited. Consequently, alternative therapies for PVT, including TIPS surgery, should be actively explored.
The theoretical benefit of TIPS for PVT is sizeable because TIPS can effectively smooth the portal vein by endovascular manipulation, and the TIPS-induced acceleration of the portal blood flow may prevent the recurrence and extension of thrombosis and its secondary complications [15, 16]; but only case reports or case series, rather than controlled studies on this topic, could be retrieved. Because of the limited data available, the comparative effectiveness of TIPS versus anticoagulation in the treatment of PVT could not be determined. In addition, a systematic review or meta-analysis was not feasible, given the heterogeneous patient population (with or without cirrhosis or malignancy), differing etiology of PVT, and the diverse indications for TIPS in the patients studied. Thus, the authors for the first time undertake a literature review to examine the feasibility and safety of TIPS in the treatment of PVT and to propose future research directions to address some issues that remain unresolved.
Methods
The PUBMED and MEDLINE (OVID) databases were searched for studies on TIPS. The reference lists of the included articles were also reviewed. The search items and eligibility criteria have been presented in “Appendix”. The last search was performed on May 1, 2011. In this manner, 445 reports were retrieved. The initial eligibility assessment was performed via a review of the title and abstract of each publication. If a final decision was not reached after this review, the full text was considered.
Overview
Sixty-five full-text articles regarding the treatment of PVT by TIPS were identified. Eleven of these articles were non-English [17–27]. The remaining 54 English full-text articles in which a total of 424 patients with PVT underwent TIPS surgery were reviewed [28–81]. Of the 54 articles, 35 were case reports (<5 PVT-TIPS patients); and 19 were case series (≥5 PVT-TIPS patients) (Table 1, 2). The TIPS insertion success rate ranged from 67 to 100% in the 19 case series. Notably, TIPS surgery for PVT was found to have been feasible in many countries (Fig. 1 ). Of the 54 studies reviewed, 52% were performed in Europe, 30% in America, and 18% in Asia. Further, an increasing trend in the number of PVT patients undergoing TIPS surgery was identified (Fig. 2). This inspiring tendency is attributed to advances in TIPS techniques and a growing awareness that TIPS may represent an important alternative therapy for PVT, especially in patients with chronic PVT and symptomatic portal hypertension in whom anticoagulation or thrombolysis has failed or is contraindicated, or in whom percutaneous portal venous recanalization and thrombectomy to maintain portal venous patency have proven ineffective.
Therapeutic strategies
Three major therapeutic strategies of TIPS surgery are used for the treatment of PVT.
The first is to initially create a portosystemic shunt via a transjugular approach, and subsequently resolve portal venous occlusion via the shunt using a balloon catheter to dislodge thrombotic material or by using local thrombolysis [31, 63, 72, 80]. The major benefit of this strategy is that the creation of a portosystemic shunt provides a direct transjugular route for portal vein recanalization [31]. With this technique, the success rate of TIPS insertion can reach approximately 100%. However, not all patients with successful TIPS insertion achieve portal vein recanalization [63, 80]. This phenomenon is primarily due to the absence of adequate blood flow into the shunt as the occluded superior mesenteric vein (SMV) fails to be recanalized (Fig. 3). Therefore, the central issue with this technique is the identification of patients in whom portal vein recanalization will not be achieved and the avoidance of unnecessary TIPS insertions in such patients.
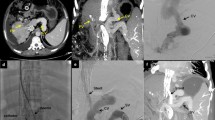
Unnecessary stent-placement in a patient with extensive thrombosis within the SMV branches. Direct portography via a percutaneous transhepatic approach showed diffuse thrombosis within the portal venous system (panel A). After a stent was successfully created, there was still diffuse thrombosis within the SMV branches (panel B). One month later, color Doppler ultrasonography revealed that the shunt was completely occluded. Indirect portography showed no blood flow through the shunt and the development of cavernous vessels (panel C). An attempt to recanalize the thrombosed shunt failed (panel D). Thick arrows indicate extensive thrombosis within the SMV, thin arrows indicate stent, dashed arrows indicate numerous collateral vessels. TH transhepatic approach
Luca et al. [80] for the first time concluded that thrombosis within a single vein, portal vein stenosis <25%, de novo diagnosis of PVT, and absence of gastroesophageal varices could independently predict a higher rate of portal vein recanalization after successful TIPS insertions. However, the clinical significance of these independent predictors is still a matter of discussion [82]. For example, in the aforementioned study, only 3% (2/70) of patients presented with portal cavernoma, and 44% (31/70) of patients presented with <50% of PVT. These inclusion biases increase the rates of TIPS success and portal vein recanalization, thereby lowering the clinical significance of predictors for portal recanalization after TIPS placement. Accordingly, further studies are necessary to accurately identify patients in whom portal vein recanalization cannot be achieved.
The second strategy is to recanalize the thrombosed portal vein via percutaneous approaches followed by TIPS placement [28, 37, 40, 52, 53, 56, 61, 78, 79]. Not all of these procedures are followed with TIPS insertions because the thrombosed portal trunk may not be successfully recanalized. Thus, unnecessary TIPS placements can be avoided. The TIPS insertion failure rate is higher in studies reporting this strategy, because a higher proportion of the included patients presented with completely occluded or obliterated main portal vein (MPV) and portal cavernoma, thereby increasing the technical difficulty. Therefore, it appears to be very necessary to identify potential patients in whom TIPS cannot be successfully placed.
To date, predictions of TIPS technical failure have been conducted in only two studies [56, 78]. Senzolo et al. demonstrated that the absence of a visible patent intrahepatic portal branch was the only risk factor for technical failure in a univariate analysis [56]. But several limitations influenced the result. First, only transjugular approaches were employed in this study. If percutaneous transhepatic approaches had been employed, some cases of technical failures might have been successful [28]. Second, this study indicated that the degree (partial or complete occlusion) and age of the PVT were not significantly associated with technical failure; but the age of thrombus was unknown in 36% of patients (10/28). In addition, TIPS placement was successful in all patients with partial PVT, and all patients in the TIPS failure group presented with total PVT. Contrarily, our team concluded that the degree of PVT was an independent predictor of TIPS technical failure in a multivariate analysis [78]. Further, TIPS procedures were recommended in patients with partially or completely occluded MPV, but not in those with obliterated MPV or fibrotic cord. This limited experience should be further confirmed in larger studies.
The third strategy is to create a TIPS between a hepatic vein and a large collateral vessel, with no need of recanalization of the thrombosed portal vein [30, 38, 51, 56, 64, 69, 78] (Table 3). This novel strategy provides an additional opportunity to divert blood from the liver and subsequently result in portal decompression in cases where a completely occluded or fibrotic portal vein cannot be recanalized. However, a large-caliber target collateral vessel that can fulfill the role of the occluded or fibrotic portal vein and be used as a stent is an essential prerequisite for this strategy. In addition, although no severe procedure-related complications occurred in these reports, precise pre-TIPS assessment of portal venous anatomy and post-TIPS surveillance are a must when undertaking this technique, and these might be not possible in all patients.
Figure 4 shows an algorithm used at our center to facilitate the TIPS procedure in the presence of PVT.
Algorithm to facilitate TIPS procedures in the treatment of portal vein thrombosis. A large collateral vessel is defined as one that can fulfill the role of the occluded or fibrotic portal vein and be used as a stent. HV hepatic vein, MPV main portal vein, PV portal vein, SMV superior mesenteric vein, TIPS transjugular intrahepatic portosystemic shunt
Approaches to access the portal vein in the presence of PVT
Four approaches are used to access the target portal vein and to further facilitate recanalization of the thrombosed portal vein (Fig. 5). These include a transjugular approach, a transhepatic approach [28, 30, 37, 39, 40, 49, 50, 52, 57, 61, 66, 70, 78], a transsplenic approach [52, 64, 69, 78], and a transmesenteric approach [34, 35, 52, 60] in order of increasing operative risk and technical difficulty.
Although a transjugular approach is safer and easier than the other three approaches, it is difficult to target a landing site in cases in which the portal vein branch is poorly visualized or in which puncture of hepatic vascular anatomy is difficult. In comparison, a transhepatic approach can provide a short and more direct access to the intrahepatic portal vein branch, a better angle for endovascular manipulations, and an easier handle for probing a thrombus. Indeed, a transjugular approach in combination with a transhepatic approach can significantly increase the portal vein recanalization rate over that achievable by using a transjugular approach alone; further, the combination does not lead to bleeding complications as the tract is embolized with a gelatin sponge [28]. However, the potential risk of bleeding from the puncture tract should be fully recognized, and emergency surgery should be adopted in a timely manner if uncontrollable intraperitoneal bleeding occurs. If puncture of the intrahepatic portal vein branch is impossible via a transhepatic approach, a transsplenic or transmesenteric approach to access the portal vein can be attempted. A patent splenic vein and additional minilaparotomy are required for the transsplenic and transmesenteric approach, respectively.
TIPS in the presence of portal cavernoma
The development of PVT is a dynamic process, ranging from recent thrombus to portal cavernoma [83]. Portal cavernoma, also known as cavernous transformation of the portal vein, refers to the formation of numerous hepatopedal collateral vessels in the liver hilum as an important compensatory mechanism for PVT [84]. At the stage of portal cavernoma, the primary therapeutic goals are to prevent and treat complications of portal hypertension and portal biliopathy [10]. Accordingly, TIPS, by decreasing portal pressure, seems to be a theoretically effective therapeutic tool in patients with either repeated variceal bleeding or refractory biliary complications. However, in the case of a portal cavernoma, portal vein puncture becomes more difficult owing to the complex anatomy, and TIPS is often contraindicated [85].
In the studies reviewed, at least 85 patients with portal cavernoma underwent successful TIPS insertions [28, 30, 33, 34, 36, 38, 40, 42, 43, 46, 51–53, 55–58, 61, 63, 64, 69, 76–80]. Two studies revealed that the rates of technical success were not significantly different between patients with and without portal cavernoma (6/9 vs. 13/19 and 3/4 vs. 10/11) [56, 61]. These results suggest that portal cavernoma should not be a contraindication for TIPS. A careful pre-operative evaluation of the portal venous system should be conducted to determine the best puncture route and to avoid the surrounding cavernous lesions [43]. In addition, as recanalization of a completely occluded or obliterated MPV is nearly impossible, TIPS insertion in a large collateral vessel, if present, can be attempted.
TIPS in candidates for liver transplantation
PVT occurs frequently in patients with advanced liver disease awaiting liver transplantations [86–88]. This poses a formidable challenge to liver transplantation because of the associated operative complexity, postoperative complications, and perioperative mortality [87–90]. Despite advances in surgical techniques, some patients with end-stage liver disease and concomitant extensive PVT are still precluded from the transplant list [91, 92]. At some centers, TIPS insertion to maintain portal vein patency is indicated for candidates for liver transplantation without any severe complications of portal hypertension (i.e., variceal bleeding and refractory ascites) [47, 63, 71, 73].
However, the extension of a stent distally into the extrahepatic MPV or the SMV can potentially jeopardize the transplant surgery [93]. Thus, whether or not TIPS stents should be extended into the SMV is debatable [94]. Stent placement primarily depends on the extension and degree of the thrombus and on whether the thrombus within the SMV could disappear if the stent was not placed into the SMV. First, if the residual SMV thrombus is slim and blood flow from the SMV into the shunt is present, the thrombus will naturally disappear after successful TIPS creation. This is primarily due to the so-called scouring effect from the persistent portal vein inflow [95]. In this case, the extension of a stent into the SMV is unnecessary. Second, if the SMV thrombus is enormous and there is little or no blood flow from the SMV into the shunt, another stent should be placed into the SMV to maintain shunt patency. In this case, the transplant surgery does become more complicated. On the one hand, if the stent were not extended to the SMV, shunt patency might be compromised. On the other hand, SMV thrombosis will greatly preclude the possibility of liver transplantation. Third, in the presence of diffuse thrombosis within the SMV branches, stent extension into the SMV is unnecessary primarily because of the absence of adequate blood flow into the shunt.
Complications of TIPS in the treatment of PVT
Procedure-related complications
Portal vein puncture and percutaneous mechanical manipulation are more dangerous in patients with PVT than in patients with normal portal venous systems. Laceration of the portal vein or liver capsule appears to be more frequent in the former patients. However, a retrospective study revealed a similar incidence of intraperitoneal hemorrhage (0% in group with PVT versus 1% in group without PVT, p = 1.00) [76]. In addition, it is important to note that intra-abdominal hemorrhage secondary to hepatic capsule perforation was lethal in three patients [39, 78, 79], as the risk of this complication was not fully recognized. These fatalities suggest that careful postoperative surveillance and timely surgical repair should be actively performed.
The most risky procedural complication that hepatologists and radiologists are concerned about is the potential risk of fatal pulmonary embolism after a portocaval shunt is successfully established, because a residual thrombus within the portal venous system may drift into the pulmonary circulation through the shunt [78, 80]. It should be noted that no episode of clinically evident pulmonary embolism has been reported in the literature yet. This is probably because the thrombus reduces in size with swift blood flow and pulmonary microembolism does not result in any clinical event. Certainly, further studies are needed to assess the possibility of pulmonary embolization after TIPS by pulmonary imaging and to explore the necessity of anticoagulant therapy for the prevention of such adverse events.
Other procedure-related complications are often reversible, including migration of a stent in the MPV, hemobilia, biliary leak from an intrahepatic duct, and hematoma in the neck [40, 61, 76, 80].
Shunt dysfunction
The overall incidence of shunt dysfunction ranged from 8 to 33% for bare stents in 13 case series [28, 31, 39, 40, 52, 53, 56, 61, 63, 72, 76, 77, 79]. 1- and 2-year cumulative rates of shunt dysfunction were reported in two case series [78, 80], but were significantly different between the two (38% vs. 21% at one postoperative year; 85% vs. 32% at two postoperative years) [78, 80]. The relatively higher rate of shunt dysfunction in the study by Luca et al. [80] might be due to the fact that anticoagulation was not used, given the possibility of anticoagulant-related hemorrhagic complications. However, no episode of such complications was observed in our study although all patients received anticoagulation after TIPS insertions. Further studies might be necessary to explore the risk-to-benefit ratio of anticoagulation for the prevention of shunt dysfunction in PVT-TIPS patients.
Compared to bare stents, covered stent-grafts can significantly improve TIPS patency [96]. Luca et al. reported that the rate of shunt dysfunction was significantly lower in patients receiving covered stents than those receiving bare stents (21% vs. 38% at one postoperative year, 29% vs. 85% at two postoperative years, p < 0.001) [80]. This finding indicates that covered stents should be recommended in cirrhotic patients with PVT.
Given that coagulation disorders are frequently observed in cirrhotic patients with PVT [97, 98], these patients might have a substantially higher risk of venous thromboembolism than those without PVT. Accordingly, the incidence of shunt thrombosis is expected to be higher in these patients than in those without PVT. However, Perarnau et al. [76] reported that the incidence of shunt stenosis in cirrhotic patients with PVT was similar to that in patients without PVT (28% vs. 35%, p = 0.57). This finding suggests that the presence of PVT does not increase the rate of shunt dysfunction in cirrhotic patients undergoing TIPS surgery.
Hepatic encephalopathy
The overall incidence of hepatic encephalopathy ranged from 0% to 50% in 10 case series [28, 31, 52, 53, 56, 61, 63, 72, 77, 79]. Nearly all episodes of hepatic encephalopathy occurred with the first postoperative year. The 1- and 2-year cumulative rates of hepatic encephalopathy were 25–27% and 27–32%, respectively in three case series [76, 78, 80]. In addition, the probability of de novo hepatic encephalopathy after TIPS was not significantly different between the patients with and without PVT (25% vs. 21% at 6 postoperative months, 27% vs. 24% at one postoperative year, 27% vs. 29% at two postoperative years, p = 0.42) [76].
Conclusions and future directions
The reviewed studies uniformly support the feasibility and safety of TIPS in the treatment of PVT, if indicated. However, the common limitations of these studies are obvious, including the retrospective nature, the absence of comparative effectiveness, the heterogeneous population, and the potential publication bias against negative studies. Thus, several future directions are further implied in this review. First, although a high rate of portal vein recanalization has been reported in PVT-TIPS patients, the long-term outcomes of such patients remain unknown. The clinical effectiveness and survival benefits of TIPS in the treatment of PVT should be further explored in prospective cohort studies. Second, TIPS has been recommended as the second-line therapeutic modality or rescue therapy for severe complications of portal hypertension in cirrhotic patients without PVT. However, given that PVT negatively influences the prognosis of cirrhotic patients, future studies should explore whether TIPS can be used as the first-line therapeutic modality in the setting of PVT. To date, only one randomized controlled trial to compare TIPS with endoscopic treatment combined with non-selective blockers and anticoagulants for the prevention of variceal rebleeding in cirrhotic patients with PVT has been registered with ClinicalTrials.gov (NCT01326949). In the future, more prospective controlled studies should be performed to compare the outcomes of TIPS with those of conservative therapy in patients with PVT. Third, although an algorithm to facilitate TIPS procedures has been developed, it should not be widely used until more practical experience is available. Further studies should focus on evaluating the benefits and drawbacks of the various TIPS techniques, especially those associated with the transsplenic and transmesenteric approaches.
Abbreviations
- AASLD:
-
American Association for the Study of Liver Diseases
- MPV:
-
Main portal vein
- PVT:
-
Portal vein thrombosis
- SMV:
-
Superior mesenteric vein
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
References
Rossle M, Richter GM, Noldge G, et al. Performance of an intrahepatic portocaval shunt (PCS) using a catheter technique: A case report. Hepatology 1988;8:1348
Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005;41:386–400
Luca A, D’Amico G, La Galla R, Midiri M, Morabito A, Pagliaro L. TIPS for prevention of recurrent bleeding in patients with cirrhosis: Meta-analysis of randomized clinical trials. Radiology 1999;212:411–21
Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis of individual patient data. Gastroenterology 2007;133:825–834
Garcia-Pagan JC, Heydtmann M, Raffa S, et al. TIPS for Budd-Chiari syndrome: Long-term results and prognostics factors in 124 patients. Gastroenterology 2008;135:808–815
Darwish Murad S, Plessier A, Hernandez-Guerra M, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med 2009;151:167–75
Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update. Hepatology 2010;51:306
Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010;362:2370–2379
DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology 2009;49:1729–1764
Sarin SK, Sollano JD, Chawla YK, et al. Consensus on extra-hepatic portal vein obstruction. Liver Int 2006;26:512–519
Condat B, Pessione F, Denninger MH, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: Increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000;32:466–470
Amitrano L, Guardascione MA, Scaglione M, et al. Prognostic factors in noncirrhotic patients with splanchnic vein thromboses. Am J Gastroenterol 2007;102:2464–2470
Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: A prospective multicenter follow-up study. Hepatology 2010;51:210–218
Thatipelli MR, McBane RD, Hodge DO, Wysokinski WE. Survival and recurrence in patients with splanchnic vein thromboses. Clin Gastroenterol Hepatol 2010;8:200–205
Riggio O, Ridola L, Lucidi C, Angeloni S. Emerging issues in the use of transjugular intrahepatic portosystemic shunt (TIPS) for management of portal hypertension: Time to update the guidelines? Dig Liver Dis 2010;42:462–467
Qi X, Han G, Fan D. The preferable treatment for cirrhotic portal vein thrombosis: anticoagulation or transjugular intrahepatic portosystemic shunt? Hepatology 2010;51:713–714
Honda M, Nishida H, Takashina T, et al. A case of alcoholic liver cirrhosis associated with portal vein thrombosis which was successfully treated by TIPS. Nippon Igaku Hoshasen Gakkai Zasshi 1993;53:220–222
Nakamura K, Takashima S, Hayashi S, et al. Transjugular intrahepatic portosystemic shunt–early experience in eleven liver cirrhosis patients. Nippon Shokakibyo Gakkai Zasshi 1994;91:1210–1219
Allgaier HP, Ochs A, Haag K, et al. Recurrent bleeding from colonic varices in portal hypertension. The successful prevention of recurrence by the implantation of a transjugular intrahepatic stent-shunt (TIPS). Dtsch Med Wochenschr 1995;120:1773–1776
Mann O, Haag K, Hauenstein KH, Rossle M, Pausch J. Septic portal vein thrombosis. Its successful therapy by local fibrinolysis and a transjugular portasystemic stent-shunt (TIPS). Dtsch Med Wochenschr 1995;120:1201–1206
Hanig V, Stenzel G, Rossle M. Acute portal vein thrombosis in liver cirrhosis: successful recanalization with the use of a portosystemic shunt (TIPS). Rofo 1996;165:403–405
Vogl T, Hidajat N, Schroder RJ, Felix R. Recanalization of an extensive fresh portal vein thrombosis by transjugular intrahepatic portosystemic stent-shunt (TIPS). Rofo 1999;171:163–165
Mamiya Y, Kanazawa H, Narahara Y, et al. A case of successful TIPS placement for gastrointestinal hemorrhage from portal hypertensive gastropathy due to complete portal venous thrombosis. Nippon Shokakibyo Gakkai Zasshi 2000;97:466–471
Chung WJ, Jang BK, Park KS, et al. Effect of transjugular intrahepatic portosystemic shunt for variceal bleeding in hepatocellular carcinoma patients with portal vein thrombosis. Korean J Hepatol 2005;11:157–163
Ramanampamonjy RM, Ramarozatovo LS, Bonnet F, et al. Portal vein thrombosis in HIV-infected patients: Report of four cases. Rev Med Interne 2005;26:545–548
Streitparth F, Santosa F, Milz J, et al. Transjugular intrahepatic portosystemic shunt in patients with portal vein thrombosis. Rofo 2008;180:899–905
Han GH, Meng XJ, Yin ZX, et al. Transjugular intrahepatic portosystemic shunt and combination with percutaneous transhepatic or transsplenic approach for the treatment of portal vein thrombosis with or without cavernomatous transformation. Zhonghua Yi Xue Za Zhi 2009;89:1549–1552
Radosevich PM, Ring EJ, LaBerge JM, et al. Transjugular intrahepatic portosystemic shunts in patients with portal vein occlusion. Radiology 1993;186:523–527
Bilbao JI, Longo JM, Rousseau H, et al. Transjugular intrahepatic portocaval shunt after thrombus disruption in partially thrombosed portal veins. Cardiovasc Intervent Radiol 1994;17:106–109
Bezzi M, Broglia L, Lemos AA, Rossi P. Transjugular intrahepatic portosystemic shunt in portal vein thrombosis: Role of the right gastric vein with anomalous insertion. Cardiovasc Intervent Radiol 1995;18:102–105
Blum U, Haag K, Rossle M, et al. Noncavernomatous portal vein thrombosis in hepatic cirrhosis: treatment with transjugular intrahepatic portosystemic shunt and local thrombolysis. Radiology 1995;195:153–157
Bayraktar Y, Oksuzoglu G, Balkanci F, et al. Portal hypertension due to incomplete membranous obstruction of the portal vein. J Clin Gastroenterol 1995;21:260–262
Gorgul A, Kayhan B, Dogan I, Unal S. Disappearance of the pseudo-cholangiocarcinoma sign after TIPSS. Am J Gastroenterol 1996;91:150–154
Matsui O, Yoshikawa J, Kadoya M, et al. Transjugular intrahepatic portosystemic shunt after previous recanalization of a chronically thrombosed portal vein via a transmesenteric approach. Cardiovasc Intervent Radiol 1996;19:352–355
Rozenblit G, DelGuercio LR, Savino JA, et al. Transmesenteric-transfemoral method of intrahepatic portosystemic shunt placement with minilaparotomy. J Vasc Interv Radiol 1996;7:499–506
Lang EV, Barnhart WH. Adjunct transjugular cholangiography for transjugular intrahepatic portosystemic shunt in chronic portal vein occlusion. AJR Am J Roentgenol 1997;168:570–571
Walser EM, NcNees SW, DeLa Pena O, et al. Portal venous thrombosis: percutaneous therapy and outcome. J Vasc Interv Radiol 1998;9:119–127
Yamagami T, Nakamura T, Tanaka O, Akada W, Takayama T, Maeda T. Transjugular intrahepatic portosystemic shunt after complete obstruction of portal vein. J Vasc Interv Radiol 1999;10:575–578
Ganger DR, Klapman JB, McDonald V, et al. Transjugular intrahepatic portosystemic shunt (TIPS) for Budd-Chiari syndrome or portal vein thrombosis: Review of indications and problems. Am J Gastroenterol 1999;94:603–608
Stein M, Link DP. Symptomatic spleno-mesenteric-portal venous thrombosis: Recanalization and reconstruction with endovascular stents. J Vasc Interv Radiol 1999;10:363–371
Sehgal M, Haskal ZJ. Use of transjugular intrahepatic portosystemic shunts during lytic therapy of extensive portal splenic and mesenteric venous thrombosis: Long-term follow-up. J Vasc Interv Radiol 2000;11:61–65
Morris CS, Najarian KE. Transjugular intrahepatic portosystemic shunt for bleeding stomal varices associated with chronic portal vein occlusion: Long-term angiographic, hemodynamic, and clinical follow-up. Am J Gastroenterol 2000;95:2966–2968
Kawamata H, Kumazaki T, Kanazawa H, Takahashi S, Tajima H, Hayashi H. Transjugular intrahepatic portosystemic shunt in a patient with cavernomatous portal vein occlusion. Cardiovasc Intervent Radiol 2000;23:145–149
Cwikiel W, Solvig J, Schroder H. Stent recanalization of chronic portal vein occlusion in a child. Cardiovasc Intervent Radiol 2000;23:309–311
Bayraktar Y, Ozturk MA, Egesel T, Cekirge S, Balkanci F. Disappearance of “pseudocholangiocarcinoma sign” in a patient with portal hypertension due to complete thrombosis of left portal vein and main portal vein web after web dilatation and transjugular intrahepatic portosystemic shunt. J Clin Gastroenterol 2000;31:328–332
Trevisani F, Rossi C, Losinno F, Bernardi M. Transjugular intrahepatic splenosystemic shunt in a patient with portal vein thrombosis. J Hepatol 2001;35:682
Liatsos C, Vlachogiannakos J, Patch D, et al. Successful recanalization of portal vein thrombosis before liver transplantation using transjugular intrahepatic portosystemic shunt. Liver Transpl. 2001;7:453–360
Ciccarelli O, Goffette P, Laterre PF, Danse E, Wittebolle X, Lerut J. Transjugular intrahepatic portosystemic shunt approach and local thrombolysis for treatment of early posttransplant portal vein thrombosis. Transplantation 2001;72:159–161
Chen S, Soares GM. Balloon-targeted access of right portal vein for transluminal intrahepatic portosystemic shunt creation in the setting of portal vein thrombosis J Vasc Interv Radiol 2003;14:513–4
Cwikiel W, Keussen I, Larsson L, Solvig J, Kullendorff CM. Interventional treatment of children with portal hypertension secondary to portal vein occlusion. Eur J Pediatr Surg 2003;13:312–318
Brountzos EN, Malagari K, Alexopoulou E, et al. Transjugular intrahepatic portosystemic shunt in cavernomatous portal vein occlusion. Hepatogastroenterology 2004;51:1168–1171
Bilbao JI, Elorz M, Vivas I, Martinez-Cuesta A, Bastarrika G, Benito A. Transjugular intrahepatic portosystemic shunt (TIPS) in the treatment of venous symptomatic chronic portal thrombosis in non-cirrhotic patients. Cardiovasc Intervent Radiol 2004;27:474–480
Jiang ZB, Shan H, Shen XY, et al. Transjugular intrahepatic portosystemic shunt for palliative treatment of portal hypertension secondary to portal vein tumor thrombosis. World J Gastroenterol 2004;10:1881–1884
Hur J, Lee KH, Lee JH, Yu JS, Won JY, Lee D. Stent-graft for TIPS in a hepatocellular carcinoma patient with main portal vein invasion. AJR Am J Roentgenol 2004;182:1301–1304
Wallace MJ, Madoff DC, Ahrar K, Warneke CL. Transjugular intrahepatic portosystemic shunts: Experience in the oncology setting. Cancer 2004;101:337–345
Senzolo M, Tibbals J, Cholongitas E, Triantos CK, Burroughs AK, Patch D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without cavernous transformation. Aliment Pharmacol Ther 2006;23:767–775
Walser EM, Soloway R, Raza SA, Gill A. Transjugular portosystemic shunt in chronic portal vein occlusion: Importance of segmental portal hypertension in cavernous transformation of the portal vein. J Vasc Interv Radiol 2006;17:373–378
Senzolo M, Cholongitas E, Tibballs J, et al. Relief of biliary obstruction due to portal vein cavernoma using a transjugular intrahepatic portosystemic shunt (TIPS) without the need for long-term stenting. Endoscopy 2006;38:760–767
Al Hamad A, Kabbani A, Al Kadhi Y. N-butyl-2-cyanoacrylate (Histoacryl) complication: a case report. Ann Saudi Med 2006;26:71–72
Yamagami T, Takeuchi Y, Sonoyama T, et al. Non-cavernomatous superior mesenteric thrombosis successfully recanalized with interventional radiological procedures carried out with a combination transmesenteric and transjugular approaches. Australas Radiol 2006;50:495–499
Van Ha TG, Hodge J, Funaki B, et al. Transjugular intrahepatic portosystemic shunt placement in patients with cirrhosis and concomitant portal vein thrombosis. Cardiovasc Intervent Radiol 2006;29:785–790
Sze DY, Magsamen KE, Frisoli JK. Successful transfemoral creation of an intrahepatic portosystemic shunt with use of the Viatorr device. J Vasc Interv Radiol 2006;17:569–572
Bauer J, Johnson S, Durham J, et al. The role of TIPS for portal vein patency in liver transplant patients with portal vein thrombosis. Liver Transpl 2006;12:1544–1551
Tuite DJ, Rehman J, Davies MH, Patel JV, Nicholson AA, Kessel DO. Percutaneous transsplenic access in the management of bleeding varices from chronic portal vein thrombosis. J Vasc Interv Radiol 2007;18:1571–1575
Ferro C, Rossi UG, Bovio G, Dahamane M, Centanaro M. Transjugular intrahepatic portosystemic shunt, mechanical aspiration thrombectomy, and direct thrombolysis in the treatment of acute portal and superior mesenteric vein thrombosis. Cardiovasc Intervent Radiol 2007;30:1070–1074
Semiz-Oysu A, Keussen I, Cwikiel W. Interventional radiological management of prehepatic obstruction of [corrected] the splanchnic venous system. Cardiovasc Intervent Radiol 2007;30:688–695
Gazzera C, Righi D, Valle F, Ottobrelli A, Grosso M, Gandini G. Fifteen years’ experience with transjugular intrahepatic portosystemic shunt (TIPS) using bare stents: Retrospective review of clinical and technical aspects. Radiol Med 2009;114:83–94
Adamus R, Pfister M, Loose RWR. Enhancing transjugular intrahepatic portosystemic shunt puncture by using three-dimensional path planning based on the back projection of two two-dimensional portographs. Radiology 2009;251:543–547
Wils A, van der Linden E, van Hoek B, Pattynama PM. Transjugular intrahepatic portosystemic shunt in patients with chronic portal vein occlusion and cavernous transformation. J Clin Gastroenterol 2009;43:982–984
Lopez-Benitez R, Barragan-Campos HM, Richter GM, et al. Interventional radiologic procedures in the treatment of complications after liver transplantation. Clin Transplant 2009;23(Suppl 21):92–101
McHugh PP, Bietz GJ, Jeon H, Johnston TD, Gedaly R, Ranjan D. Transjugular intrahepatic portosystemic shunt to keep vein open. Liver Transpl 2009;15:558–560
Liu FY, Wang MQ, Fan QS, Duan F, Wang ZJ, Song P. Interventional treatment for symptomatic acute-subacute portal and superior mesenteric vein thrombosis. World J Gastroenterol 2009;15:5028–5034
Finkenstedt A, Graziadei IW, Nachbaur K, et al. Transjugular intrahepatic portosystemic shunt in liver transplant recipients. World J Gastroenterol 2009;15:1999–2004
Kav T, Peynircioglu B, Balkanci F, Bayraktar Y. Long-term patency of transjugular intrahepatic portosystemic shunt in a patient with portal vein web. J Clin Gastroenterol 2009;43:791–792
Lodhia N, Salem R, Levitsky J. Transjugular intrahepatic portosystemic shunt with thrombectomy for the treatment of portal vein thrombosis after liver transplantation. Dig Dis Sci 2010;55:529–534
Perarnau JM, Baju A, D’Alteroche L, Viguier J, Ayoub J. Feasibility and long-term evolution of TIPS in cirrhotic patients with portal thrombosis. Eur J Gastroenterol Hepatol 2010;22:1093–1098
Fanelli F, Angeloni S, Salvatori FM, et al. Transjugular intrahepatic portosystemic shunt with expanded-polytetrafuoroethylene-covered stents in non-cirrhotic patients with portal cavernoma. Dig Liver Dis 2011;43:78–84
Han G, Qi X, He C, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol 2011;54:78–88
Luo JJ, Yan ZP, Wang JH, Liu QX, Qu XD. Endovascular treatment for nonacute symptomatic portal venous thrombosis through intrahepatic portosystemic shunt approach. J Vasc Interv Radiol 2011;22:61–69
Luca A, Miraglia R, Caruso S, et al. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut 2011;60:846–852
Miraglia R, Maruzzelli L, Luca A. Transjugular intrahepatic porto-systemic shunt placement in a patient with left-lateral split-liver transplant and mesenterico-left portal vein by pass placement. Cardiovasc Intervent Radiol 2011 (in press)
Han G, Qi X, Guo W, Niu J, Bai M, Fan D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis in cirrhosis. Gut 2011 (in press)
Qi X, Han G, Bai M, Fan D. Stage of portal vein thrombosis. J Hepatol 2011;54:1080–1082
Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol 2000;32:865–871
Boyer TD, Haskal ZJ. American Association for the Study of Liver Diseases practice guidelines: the role of transjugular intrahepatic portosystemic shunt creation in the management of portal hypertension. J Vasc Interv Radiol 2005;16:615–629
Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005;54:691–697
Englesbe MJ, Kubus J, Muhammad W, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl 2010;16:83–90
Englesbe MJ, Schaubel DE, Cai S, Guidinger MK, Merion RM. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl 2010;16:999–1005
Gayowski TJ, Marino IR, Doyle HR, et al. A high incidence of native portal vein thrombosis in veterans undergoing liver transplantation. J Surg Res 1999;60:333–338
Qi X, Bai M, Yang Z, et al. Occlusive portal vein thrombosis as a new marker of decompensated cirrhosis. Med Hypotheses 2011;76:522–526
O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology 2008;134:1764–1776
Jamieson NV. Changing perspectives in portal vein thrombosis and liver transplantation. Transplantation 2000;69:1772–1774
Senzolo M, Riggio O, Primignani M. Vascular disorders of the liver: Recommendations from the Italian Association for the Study of the Liver (AISF) ad hoc committee. Dig Liver Dis 2011;43:503–514
Senzolo M, Patch D, Burra P, Burroughs AK. TIPS for portal vein thrombosis (PVT) in cirrhosis: not only unblocking a pipe. J Hepatol 2011;55:945–946
Han G, Qi X, He C, Fan D. TIPS for portal vein thrombosis (PVT): still a long way to go. J Hepatol 2011;55:947–948
Yang Z, Han G, Wu Q, et al. Patency and clinical outcomes of transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stents versus bare stents: a meta-analysis. J Gastroenterol Hepatol 2010;25:1718–1725
Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol 2010;15:116–121
Amitrano L, Brancaccio V, Guardascione MA, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology 2000;31:345–348
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 81000864) and the Chinese Postdoctoral Science Foundation (no. 20100471776).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Search items
(“Portal venous” (all fields) or “portal vein” (all fields)) and “thrombosis” (all fields) or “thrombus” (all fields) or “thrombi” (all fields) or “thrombin” (all fields) or “thrombosed” (all fields) or “thrombotic” (all fields) or “occlusion” (all fields) or “occlusive” (all fields) or “occluded” (all fields) or “obstruction” (all fields) or “obstructed” (all fields) or “stenosis” (All Fields) or “stenotic” (all fields) or “embolization” (all fields) or “embolisation” (all fields) or “embolism” (all fields) or “emboli” (all fields)) and (“transjugular intrahepatic portosystemic shunt” (all fields) or “TIPSS” (all fields)).
Eligibility criteria
Inclusion criteria
-
1.
All case reports, case series, cohort studies, and controlled studies were included, regardless of the retrospective or prospective nature of the study.
-
2.
No publication date restrictions were imposed.
-
3.
The participants were diagnosed with PVT; they included children and adults with or without underlying liver cirrhosis and with or without malignancy.
-
4.
The participants underwent TIPS procedures, and each case result was included in the study regardless of technical failure or success.
Exclusion criteria
-
1.
Reviews or comments on the treatment of PVT or the applications of TIPS were excluded.
-
2.
Abstracts and non-English language full-text articles were excluded.
-
3.
The objectives of the study were assessed in animals.
-
4.
Portal vein obstruction caused by external constriction.
-
5.
Thrombosis occurred within the portal vein as a complication of stent stenosis or hepatic vein outflow obstruction.
-
6.
Portal vein recanalization was achieved using the percutaneous approach alone.
Rights and permissions
About this article
Cite this article
Qi, X., Han, G. Transjugular intrahepatic portosystemic shunt in the treatment of portal vein thrombosis: a critical review of literature. Hepatol Int 6, 576–590 (2012). https://doi.org/10.1007/s12072-011-9324-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-011-9324-5