Abstract
Purpose
This study was conducted to prospectively investigate the interobserver reproducibility of controlled attenuation parameter (CAP) measurements and the relationship among the CAP and body mass index (BMI), gender and age.
Methods
Consecutive subjects were studied using the M+ probe of the FibroScan device (Echosens, Paris, France). Measurements were performed by two raters (rater1 and rater2). Interobserver agreement was assessed by using the concordance correlation coefficient (CCC). The Pearson r coefficient was used to test correlation between two study variables, and linear regression was used for the multivariate model.
Results
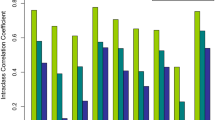
Three hundred fifty-one subjects (227 males and 124 females) were prospectively studied. The CCC was 0.82 (95 % CI 0.78–0.85) overall, 0.80 (95 % CI 0.75–0.85) for BMI <25 kg/m2, 0.76 (95 % CI 0.69–0.84) for BMI 25–29 kg/m2 and 0.65 (95 % CI 0.41–0.88) for BMI ≥30 kg/m2. The CCC was 0.44 (95 % CI 0.31–0.56) for CAP values ≤240 dB/m and 0.72 (95 % CI 0.65–0.79) for CAP values >240 dB/m. In univariate analysis, age and BMI by gender were correlated with the CAP. Multiple regression analysis confirmed the relationship of the CAP with age and BMI, but not with gender.
Conclusions
The results of this study show that the interreader agreement in CAP measurement is good. In healthy volunteers, the CAP is strongly correlated with age and BMI.


Similar content being viewed by others
References
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231
Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317
Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121
Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835
Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20
de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918
Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis usinf Fibroscan®: validation in chronic hepatitis C. J Viral Hepat. 2012;19:244–253
Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910
Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim do Y, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102–109
Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, et al. Controlled attenuation parameter for non-invasive assessment of hepaticsteatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013;28:1194–1201
Lédinghen VD, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C et al. Controlled Attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5,323 examinations. J Hepatol 2013; doi:10.1016/j.jhep.2013.12.018
Recio E, Cifuentes C, Macias J, Mira JA, Parra-Sánchez M, Rivero-Juárez A, et al. Interobserver concordance in controlled attenuation parameter measurement, a novel tool for the assessment of hepatic steatosis on the basis of transient elastography. Eur J Gastroenterol Hepatol. 2013;25:905–911
Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268
Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1997
Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B et al. Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013; 57:1182–1191
Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973
Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–448
Gilsanz V, Chung SA, Kaplowitz N. Differential effect of gender on hepatic fat. Pediatr Radiol. 2011;41:1146–1153
Denzer C, Thiere D, Muche R, Koenig W, Mayer H, Kratzer W, et al. Gender-specific prevalences of fatty liver in obese children and adolescents: roles of body fat distribution, sex steroids, and insulin resistance. J Clin Endocrinol Metab. 2009;94:3872–3881
Lee K, Sung JA, Kim JS, Park TJ. The roles of obesity and gender on the relationship between metabolic risk factors and nonalcoholic fatty liver disease in Koreans. Diabetes Metab Res Rev. 2009;25:150–155
Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905
Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161
Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13, 369 examinations. Hepatology. 2010;51:828–835
Acknowledgments
The authors would like to thank the nurses in the outpatient ward of the Infectious Diseases Department, including Ms. Livia Astroni, Ms. Natali Calabrese, Mr. Filippo Cuda, Mr. Lorenzo Guioli, Ms. Maura Marchisoni, Ms. Giampiera Nava, Ms. Loredana Pavesi and Ms. Barbara Ricci, the nurses in the “Medicina Diagnostica e dei Servizi” Department, including Ms. Paola Bolzoni, Ms. Anna Cuollo, Ms Giuseppina Discenza and Ms. Nadia Locatelli, the secretary of the Ultrasound Unit, for their valuable help in complying with the study protocol. The authors are very grateful to Magali Sasso, PhD, (Echosens, Paris) for her valuable advice on the CAP results.
Compliance with ethical requirements and Conflict of interest
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study. Giovanna Ferraioli, Carmine Tinelli, Raffaella Lissandrin, Mabel Zicchetti, Mariangela Rondanelli, Guido Perani, Stefano Bernuzzi, Laura Salvaneschi and Carlo Filice declare that they have no conflict of interest. The members of the “Liver Steatosis Study Group” Elisabetta Above, Giorgio Barbarini, Raffaele Bruno, Silvia Corona, Carolina Dellafiore, Marta Di Gregorio, Roberto Gulminetti, Paolo Lanzarini, Serena Ludovisi, Laura Maiocchi, Antonello Malfitano, Giuseppe Michelone, Lorenzo Minoli, Mario Mondelli, Stefano Novati, Savino F.A. Patruno, Alessandro Perretti, Gianluigi Poma, Paolo Sacchi, Domenico Zanaboni and Marco Zaramella declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Liver Steatosis Study Group.
Liver Steatosis Study Group
Elisabetta Above, MD, Giorgio Barbarini, MD, Raffaele Bruno, MD, Silvia Corona, MSc, Carolina Dellafiore, MD, Marta Di Gregorio, MD, Roberto Gulminetti, MD, Paolo Lanzarini, MD, Serena Ludovisi, MD, Laura Maiocchi, MD, Antonello Malfitano, MD, Giuseppe Michelone, MD, Lorenzo Minoli, MD, Mario Mondelli, MD, Stefano Novati, MD, Savino F.A. Patruno, MD, Alessandro Perretti, MD, Gianluigi Poma, MD, Paolo Sacchi, MD, Domenico Zanaboni, MD, and Marco Zaramella, MD.
Rights and permissions
About this article
Cite this article
Ferraioli, G., Tinelli, C., Lissandrin, R. et al. Interobserver reproducibility of the controlled attenuation parameter (CAP) for quantifying liver steatosis. Hepatol Int 8, 576–581 (2014). https://doi.org/10.1007/s12072-014-9573-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-014-9573-1