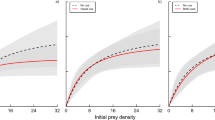
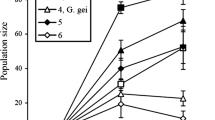
Abstract
Biotic resistance from native predators can play an important role in regulating or limiting exotic prey. We investigate how global warming potentially alters the strength and spatial extent of these predator–prey interactions in aquatic insect ecosystems. As a simple model system, we use rock pools in streams of rainforests of Hawaii, which contain the beautiful Hawaiian damselfly Megalagrion calliphya as predator and the invasive southern house mosquito Culex quinquefasciatus as prey. This abundant mosquito is the major vector of avian malaria transmission to native forest birds. We use mathematical modeling to evaluate the potential impacts of damselfly predation and temperature on mosquito population dynamics. We model this predator–prey system along an elevational gradient (749-1952 m elevation) and assess the effect of 1°C and 2°C climate warming scenarios as well as the effects of El Niño and La Niña oscillations, on predator–prey dynamics. Our results indicate that the strength of biotic resistance of native predators on invasive prey may decrease with increasing temperature because demographic rates of predator and prey are differentially affected by temperature. Future warming could therefore increase the abundance of invasive species by releasing them from predation pressure. If the invasive species is a disease vector, these shifts could increase the impact of disease on both humans and wildlife.






Similar content being viewed by others
References
Ahumada JA, Lapointe D, Samuel MD (2004) Modeling the population dynamics of Culex quinquefasciatus (Diptera: Culicidae), along an elevational gradient in Hawaii. J Med Entomol 41(6):1157–1170. doi:10.1603/0022–2585–41.6.1157
Ahumada JA, Samuel MD, Duffy DC, Dobson A, Hobbelen PHF (2009) Modeling the epidemiology of avian malaria and pox in Hawaii. In: Pratt TK, Atkinson CT, Banko PC, Jacobi J, Woodworth BL (eds) Hawaiian forest birds: their biology and conservation. Yale University Press, New Haven, pp 331–355
Anderson D, Watson R (1980) On the spread of a disease with gamma-distributed latent and infectious period. Biometrika 67(1):191–198
Atkinson CT, Woods KL, Dusek RJ, Sileo LS, Iko WM (1995) Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected Iiwi (Vestiaria coccinea). Parasitology 111:S59–S69. doi:10.1017/S003118200007582X
Benning TL, LaPointe D, Atkinson CT, Vitousek PM (2002) Interactions of climate change with biological invasions and land use in the Hawaiian Islands: modeling the fate of endemic birds using a geographic information system. Proc Natl Acad Sci USA 99(22):14246–14249. doi:10.1073/pnas.162372399
Benstead JP, March JG, Pringle CM, Ewel KC, Short JW (2009) Biodiversity and ecosystem function in species-poor communities: community structure and leaf litter breakdown in a Pacific island stream. J North Am Benthol Soc 28(2):454–465. doi:10.1899/07–081.1
Brown JH, Gillooly J, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. doi:10.1890/03–9000
Cain ML, Bowman WD, Hacker SD (2011) Ecology, 2nd edn. Sinauer, Sunderland, MA
Carlsson NOL, Sarnelle O, Strayer DL (2009) Native predators and exotic prey—an acquired taste? Frontiers Ecology Environ 7(10):525–532. doi:10.1890/080093
Carlsson NOL, Bustamante H, Strayer DL, Pace ML (2011) Biotic resistance on the increase: native predators structure invasive zebra mussel populations. Freshwater Biol 56:1630–1637. doi:10.1111/j.1365–2427.2011.02602.x
Caswell H (2001) Matrix population models, 2nd edn. Sinauer, Sunderland, MA
Cooper G, Holland PWH, Miller PL (1996) Captive breeding of Ischnura elegans (Van der Linden): observations on longevity, copulation and oviposition (Zygoptera: Coenagrionidae). Odonatologica 25:261–273
Corbet PS (1999) Dragonflies: behaviour and ecology of Odonata. Cornell University Press, Ithaca, NY
De Block M, Stoks R (2005) Fitness effects from egg to reproduction: bridging the life history transition. Ecology 86(1):185–197. doi:10.1890/04–0116
deRivera CE, Ruiz GM, Hines AH, Jivoff P (2005) Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology 86(12):3364–3376. doi:10.1890/05–0479
Dumont CP, Gaymer CF, Thiel M (2011) Predation contributes to invasion resistance of benthic communities against the non-indigenous tunicate Ciona intestinalis. Biol Invasions 13:2023–2034
Elton CS (1958) Ecology of invasions by animals and plants. Methuen, London
Eyre MD, Woodward JC, Luff ML (2005) Expanding northern ranges of aquatic invertebrate species: a possible effect of climate change? Brit J Entomol Nat Hist 18:219–223
Feng Z, Xu D, Zhao H (2007) Epidemiological models with non-exponentially distributed disease stages and applications to disease control. Bull Math Biol 69(5):1511–1536. doi:10.1007/s11538–006–9174–9
Forbes M, Leung B (1995) Pre-fabricated dining shelters as outdoor insectaries, an assessment using Enallagma ebrium (Hagen) (Zygoptera: Coenagrionidae). Odonatologica 24(4):461–466
Giambelluca TW, Diaz HF, Luke MSA (2008) Secular temperature changes in Hawaii. Geophysical Research Letters 35:L12702. doi:10.1029/2008GL034377
Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH (2002) Effects of size and temperature on developmental time. Nature 417(6884):70–73. doi:doi:10.1038/417070a
Gruner DS (2005) Biotic resistance to an invasive spider conferred by generalist insectivorous birds on Hawai’i Island. Biol Invasions 7(3):541–546. doi:10.1007/s10530–004–2509–2
Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296(5576):2158–2162. doi:10.1126/science.1063699
Hayes J, Hsi BP (1975) Interrelationships between selected meteorological phenomena and immature stages of Culex pipiens quinquefasciatus Say—study of an isolated population. J Med Entomol 12(3):299–308
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biol 12(3):450–455. doi:10.1111/j.1365–2486.2006.01116.x
Hinnekint BON (1987) Population dynamics of Ischnura elegans Van der Linden (Insecta: Odonata) with special reference to morphological color changes, female polymorphism, multiannual cycles and their influence on behaviour. Hydrobiologia 146(1):3–31
Holling CS (1959) Components of predation as revealed by a study of small-mammal predation of the European sawfly. Can Entomol 91:293–320
Jacobi JD, Atkinson CT (1995) Hawaii’s endemic birds. In: LaRoe ET, Farris GS, Puckett CE, Doran PD, Mac MJ (eds) Our living resources: a report to the nation on the distribution, abundance and health of US plants, animals and ecosystems. United States Department of the Interior, National Biological Service, Washington, DC, pp 376–381
LaPointe DA (2000) Avian malaria in Hawaii: the distribution, ecology and vector potential of forest-dwelling mosquitoes. University of Hawaii, Honolulu, HI, Dissertation
LaPointe DA, Goff ML, Atkinson CT (2010) Thermal constraints on the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai’i. J Parasitol 96(2):318–324. doi:10.1645/ge-2290.1
Lloyd AL (2001) Destabilization of epidemic models with the inclusion of realistic distributions of infectious periods. Proc R Soc Lond B Biol Sci 268(1470):985–993. doi:10.1098/rspb.2001.1599
Logan JD, Wolesensky W, Joern A (2006) Temperature-dependent phenology and predation in arthropod systems. Ecol Model 196(3–4):471–482. doi:10.1016/j.ecolmodel.2006.02.034
Loope LL, Giambelluca TW (1998) Vulnerability of island tropical montane cloud forests to climate change, with special reference to East Maui, Hawaii. Clim Change 39(2–3):503–517. doi:10.1023/A:1005372118420
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10(3):689–710. doi:10.2307/2641039
Montoya JM, Raffaelli D (2010) Climate change, biotic interactions and ecosystem services. Phil Trans R Soc B Biol Sci 365(1549):2013–2018. doi:10.1098/rstb.2010.0114
Musolin DL (2007) Insects in a warmer world: ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Global Change Biol 13(8):1565–1585. doi:10.1111/j.1365–2486.2007.01395.x
Rogers D (1972) Random search and insect population models. J Anim Ecol 41(2):369–383
Samuel MD, Hobbelen PHF, DeCastro F, Ahumada JA, LaPointe DA, Atkinson CT, Woodworth BL, Hart PJ, Duffy DC (2011) The dynamics, transmission, and population impacts of avian malaria in native Hawaiian birds: a modeling approach. Ecol Appl 21:2960–2973
Savage VM, Gillooly JF, Brown JH, West GB, Charnov EL (2004) Effects of body size and temperature on population growth. Am Nat 163:429–441. doi:dio:10.1086/381872
Schenk D, Bacher S (2002) Functional response of a generalist insect predator to one of its prey species in the field. J Anim Ecol 71(3):524–531. doi:10.1046/j.1365–2656.2002.00620.x
Thieme HR (2003) Mathematics in population biology. Princeton University Press, Princeton, NJ, Princeton series in theoretical and computational biology
Thompson DJ (1978) Towards a realistic predator–prey model: the effect of temperature on the functional response and life history of larvae of the damselfly, Ischnura elegans. J Anim Ecol 47(3):757–767
Trottier R (1971) Effect of temperature on the life-cycle of Anax junius (Odonata: Aeshnidae) in Canada. Can Entomol 103(12):1671–1683
Tylianakis JM (2009) Warming up food webs. Science 323(5919):1300–1301. doi:10.1126/science.1170909
Van Riper C III (1991) The impact of introduced vectors and avian malaria on insular passeriform bird populations in Hawaii. Bull Soc Vector Ecol 16(1):59–83
Van Riper C III, Van Riper SG, Goff ML, Laird M (1986) Epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr 56(4):327–344
Veiga P, Rubal M, Arenas F, Incera M, Olabarria C, Sousa-Pinto I (2011) Does Carcinus maenas facilitate the invasion of Xenostrobus secures? J Exp Mar Biol Ecol 406:14–20
Visser ME, Both C (2005) Shifts in phenology due to global climate change: the need for a yardstick. Proc R Soc Lond B Biol Sci 272(1581):2561–2569. doi:10.1098/rspb.2005.3356
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416(6879):389–395. doi:10.1038/416389a
Walther GR, Roques A, Hulme PE, Sykes MT, Pysek P, Kuhn I, Zobel M, Bacher S, Botta-Dukat Z, Bugmann H, Czucz B, Dauber J, Hickler T, Jarosik V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vila M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24(12):686–693. doi:10.1016/j.tree.2009.06.008
Williams FX (1936) Biological studies in Hawaiian water-loving insects. Part II. Odonata or Dragonflies. Proc Hawaii Entomol Soc 9:273–348
Woodworth BL, Atkinson CT, LaPointe DA, Hart PJ, Spiegel CS, Tweed EJ, Henneman C, LeBrun J, Denette T, DeMots R, Kozar KL, Triglia D, Lease D, Gregor A, Smith T, Duffy D (2005) Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc Natl Acad Sci USA 102(5):1531–1536. doi:10.1073/pnas.0409454102
Acknowledgments
We would like to thank JA Ahumada and F. de Castro for their contribution to the development of the mosquito model and F. de Castro for his help in the generation of daily temperature data for the Pahala watershed. We also thank MV Martinez for his help with the preparation of climate data files for the program ANUSPLIN. This project was supported by funding through the US Geological Survey Climate Change Research Initiative and additional funding through the Invasive Species and Terrestrial Wildlife Programs of the US Geological Survey. Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hobbelen, P.H.F., Samuel, M.D., Foote, D. et al. Modeling the impacts of global warming on predation and biotic resistance: mosquitoes, damselflies and avian malaria in Hawaii. Theor Ecol 6, 31–44 (2013). https://doi.org/10.1007/s12080-011-0154-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-011-0154-9