Abstract
Purpose
Thiamine-dependent enzymes (TDEs) linking glycolysis with the tricarboxylic acid cycle (TCA) pyruvate dehydrogenase (PDH), of the pentose phosphate pathway transketolases (TKTs), the TCA alpha-ketoglutarate deydrogenase (KGDH)/2-oxoglutarate dehydrogenase (OGDH) complex, and the amino acid catabolism branched-chain alpha-ketoacid dehydrogenase (BCKDH) complex are crucial factors for tumor metabolism. The expression of these enzymes has not been analyzed for carcinogenesis of oral squamous cell carcinoma (OSCC) with special focus on new targeted metabolic therapies as yet.
Methods
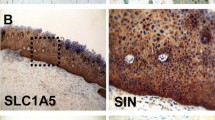
TDEs PDH, KGDH (OGDH), and BCKDH were analyzed in normal oral mucosa (n = 14), oral precursor lesions (simple hyperplasia, n = 21; squamous intraepithelial neoplasia, SIN I–III, n = 35), and OSCC specimen (n = 46) by immunohistochemistry and western blot (WB) analysis in OSCC tumor cell lines.
Results
Although the total numbers of PDH and KGDH (OGDH) positive samples decreased in OSCC, both enzymes were significantly overexpressed in the carcinogenesis of OSCC compared with normal tissue. BCKDH has been demonstrated to be significantly overexpressed in the carcinogenesis of OSCC. Specificity of the antibodies was confirmed by WB analysis.
Conclusions
This is the first study showing increased expression of TDEs in OSCC. Metabolic targeting of TDEs (including TKTs) by antagonistic compounds like oxythiamine or oxybenfothiamine may be a useful strategy to sensitize cancer cells to common OSCC cancer therapies.







Similar content being viewed by others
Abbreviations
- SIN:
-
Squamous intraepithelial neoplasia
- OSCC:
-
Oral squamous cell carcinoma
- PDH:
-
Pyruvate dehydrogenase
- PPP:
-
Pentose phosphate pathway
- TKT:
-
Transketolase
- TCA:
-
Tricarboxylic acid cycle
- KGDH:
-
Ketoglutarate dehydrogenase
- OGDH:
-
2-oxoglutarate deydrogenase
- BCKDH:
-
Branched-chain alpha-ketoacid dehydrogenase
- TCA:
-
Tricarboxylic acid cycle
References
Grimm M, Cetindis M, Lehmann M, Biegner T, Munz A, Teriete P, et al. Association of cancer metabolism-related proteins with oral carcinogenesis—indications for chemoprevention and metabolic sensitizing of oral squamous cell carcinoma? J Transl Med. 2014;12:208. doi:10.1186/1479-5876-12-208.
Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Investig. 2013;123(9):3678–84. doi:10.1172/JCI69600.
Alfarouk KO, Shayoub ME, Muddathir AK, Elhassan GO, Bashir AH. Evolution of Tumor Metabolism might reflect carcinogenesis as a reverse evolution process (dismantling of multicellularity). Cancers Basel. 2011;3(3):3002–17. doi:10.3390/cancers3033002.
Cetindis M, Biegner T, Munz A, Teriete P, Reinert S, Grimm M. Glutaminolysis and carcinogenesis of oral squamous cell carcinoma. Eur Arch Oto Rhino Laryngol Off J Eur Fed Oto Rhino Laryngol Soc. 2015;. doi:10.1007/s00405-015-3543-7.
Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3(1):1. doi:10.1186/s40170-015-0128-2.
Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol Lett. 2012;4(6):1151–7. doi:10.3892/ol.2012.928.
Ramos-Montoya A, Lee WN, Bassilian S, Lim S, Trebukhina RV, Kazhyna MV, et al. Pentose phosphate cycle oxidative and nonoxidative balance: a new vulnerable target for overcoming drug resistance in cancer. Int J Cancer J Int Du Cancer. 2006;119(12):2733–41. doi:10.1002/ijc.22227.
Grimm M, Schmitt S, Teriete P, Biegner T, Stenzl A, Hennenlotter J, et al. A biomarker based detection and characterization of carcinomas exploiting two fundamental biophysical mechanisms in mammalian cells. BMC Cancer. 2013;13(1):569. doi:10.1186/1471-2407-13-569.
Grimm M, Munz A, Teriete P, Nadtotschi T, Reinert S. GLUT-1(+)/TKTL1(+) coexpression predicts poor outcome in oral squamous cell carcinoma. Oral Surg Oral Med Oral pathol Oral Radiol. 2014;117(6):743–53. doi:10.1016/j.oooo.2014.02.007.
Caneba CA, Bellance N, Yang L, Pabst L, Nagrath D. Pyruvate uptake is increased in highly invasive ovarian cancer cells under anoikis conditions for anaplerosis, mitochondrial function, and migration. Am J Physiol Endocrinol Metab. 2012;303(8):E1036–52. doi:10.1152/ajpendo.00151.2012.
Zastre JA, Sweet RL, Hanberry BS, Ye S. Linking vitamin B1 with cancer cell metabolism. Cancer Metab. 2013;1(1):16. doi:10.1186/2049-3002-1-16.
Xu X, Zur Hausen A, Coy JF, Lochelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer J Int Du Cancer. 2009;124(6):1330–7. doi:10.1002/ijc.24078.
Santos CR, Schulze A. Lipid metabolism in cancer. The FEBS journal. 2012;279(15):2610–23. doi:10.1111/j.1742-4658.2012.08644.x.
Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54. doi:10.1146/annurev.nu.04.070184.002205.
Harris RA, Joshi M, Jeoung NH, Obayashi M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J Nutr. 2005;135(6 Suppl):1527S–30S.
Platell C, Kong SE, McCauley R, Hall JC. Branched-chain amino acids. J Gastroenterol Hepatol. 2000;15(7):706–17.
Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18(20):5585–94. doi:10.1158/1078-0432.CCR-12-0858.
Sattler UG, Mueller-Klieser W. The anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol. 2009;85(11):963–71. doi:10.3109/09553000903258889.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–91. doi:10.1038/nrd2803.
Tanaka T, Tanaka M, Tanaka T. Oral carcinogenesis and oral cancer chemoprevention: a review. Patholog Res Int. 2011;2011:431246. doi:10.4061/2011/431246.
Driemel O, Hertel K, Reichert TE, Kosmehl H. Current classification of precursor lesions of oral squamous cell carcinoma principles of the WHO classification 2005. Mund Kiefer Gesichtschir. 2006;10(2):89–93. doi:10.1007/s10006-006-0675-3.
Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10(4):267–77. doi:10.1038/nrc2817.
Sobin LH, Ch W, editors. UICC TNM classification of malignant tumors Berlin. 7th ed. Berlin: Springer Verlag; 2010.
Hamilton SR, Aaltonen LA. Pathology and genetics. Tumours of the digestive system. 3rd ed. Lyon: IARC Press; 2000.
Walker RA. Quantification of immunohistochemistry–issues concerning methods, utility and semiquantitative assessment I. Histopathology. 2006;49(4):406–10. doi:10.1111/j.1365-2559.2006.02514.x.
Grimm M, Munz A, Teriete P, Nadtotschi T, Reinert S. GLUT-1 +/TKTL1 + co-expression predicts poor outcome in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(6):743–53.
Edington KG, Loughran OP, Berry IJ, Parkinson EK. Cellular immortality: a late event in the progression of human squamous cell carcinoma of the head and neck associated with p53 alteration and a high frequency of allele loss. Mol Carcinog. 1995;13(4):254–65.
Alexander D, Schafer F, Olbrich M, Friedrich B, Buhring HJ, Hoffmann J, et al. MSCA-1/TNAP selection of human jaw periosteal cells improves their mineralization capacity. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2010;26(6):1073–80. doi:10.1159/000323985.
Grimm M, Alexander D, Munz A, Hoffmann J, Reinert S. Increased LDH5 expression is associated with lymph node metastasis and outcome in oral squamous cell carcinoma. Clin Exp Metastasis. 2013;30(4):529–40. doi:10.1007/s10585-012-9557-2.
Sun W, Liu Y, Glazer CA, Shao C, Bhan S, Demokan S, et al. TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(3):857–66. doi:10.1158/1078-0432.CCR-09-2604.
Liu S, Miriyala S, Keaton MA, Jordan CT, Wiedl C, Clair DK, et al. Metabolic effects of acute thiamine depletion are reversed by rapamycin in breast and leukemia cells. PLoS One. 2014;9(1):e85702. doi:10.1371/journal.pone.0085702.
Mandujano-Tinoco EA, Gallardo-Perez JC, Marin-Hernandez A, Moreno-Sanchez R. Rodriguez-Enriquez S (2013) Anti-mitochondrial therapy in human breast cancer multi-cellular spheroids. Biochim Biophys Acta. 1833;3:541–51. doi:10.1016/j.bbamcr.2012.11.013.
Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26(9):877–90. doi:10.1101/gad.189365.112.
Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98(12):1975–84. doi:10.1038/sj.bjc.6604356.
McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim N, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–8.
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, Tumor, et al. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia. 2005;7(1):1–6.
Stacpoole PW. The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell. 2012;11(3):371–7. doi:10.1111/j.1474-9726.2012.00805.x.
Stuart SD, Schauble A, Gupta S, Kennedy AD, Keppler BR, Bingham PM, et al. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab. 2014;2(1):4. doi:10.1186/2049-3002-2-4.
Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun. 2004;313(2):391–6.
Wang J, Zhang X, Ma D, Lee WN, Xiao J, Zhao Y, et al. Inhibition of transketolase by oxythiamine altered dynamics of protein signals in pancreatic cancer cells. Exp Hematol Oncol. 2013;2:18. doi:10.1186/2162-3619-2-18.
Rais B, Comin B, Puigjaner J, Brandes JL, Creppy E, Saboureau D, et al. Oxythiamine and dehydroepiandrosterone induce a G1 phase cycle arrest in Ehrlich’s tumor cells through inhibition of the pentose cycle. FEBS Lett. 1999;456(1):113–8.
Boros L, Puigjaner J, Cascante M, Lee W-N, Brandes J, Bassilian S, et al. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 1997;57:4242–8.
Hamabe A, Yamamoto H, Konno M, Uemura M, Nishimura J, Hata T, et al. Combined evaluation of hexokinase 2 and phosphorylated pyruvate dehydrogenase-E1alpha in invasive front lesions of colorectal tumors predicts cancer metabolism and patient prognosis. Cancer Sci. 2014;105(9):1100–8. doi:10.1111/cas.12487.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. doi:10.1016/j.cmet.2007.10.002.
Hurd TR, DeGennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012;22(2):107–15. doi:10.1016/j.tcb.2011.11.002.
Coy JF, Dressler D, Wilde J, Schubert P. Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin Lab. 2005;51(5–6):257–73.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–68. doi:10.1038/nrc2499.
Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8.
Campbell LL, Polyak K. Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle. 2007;6(19):2332–8. doi:10.4161/cc.6.19.4914.
Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10(3):175–6. doi:10.1016/j.ccr.2006.08.015.
Frezza C, Gottlieb E. Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol. 2009;19(1):4–11. doi:10.1016/j.semcancer.2008.11.008.
Simon H, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–8.
Grimm M. Prognostic value of clinicopathological parameters and outcome in 484 patients with oral squamous cell carcinoma: microvascular invasion (V+) is an independent prognostic factor for OSCC. Clinical Transl Oncol Off Pub Fed Spanish Oncol Soc National Cancer Inst Mexico. 2012;14(11):870–80. doi:10.1007/s12094-012-0867-2.
Perez-Sayans M, Suarez-Penaranda JM, Pilar GD, Barros-Angueira F, Gandara-Rey JM, Garcia-Garcia A. Hypoxia-inducible factors in OSCC. Cancer Lett. 2011;313(1):1–8. doi:10.1016/j.canlet.2011.08.017.
Heier M, Dornish J. Effect of the fluoropyrimidines 5-fluorouracil and doxifluridine on cellular uptake of thiamin. Anticancer Res. 1989;9:1073–7.
Aksoy M, Basu T, Brient J, Dickerson J. Thiamin status of patients treated with drug combinations containing 5-fluorouracil. Eur J Cancer. 1980;16:1041–5.
Sweet R, Paul A, Zastre J. Hypoxia induced upregulation and function of the thiamine transporter, SLC19A3 in a breast cancer cell line. Cancer Biol Ther. 2010;10:1101–11.
Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–5. doi:10.1074/jbc.M202487200M202487200.
Boros L. Population thiamine status and varying cancer rates between western, Asian and African countries. Anticancer Res. 2000;20:2245–8.
Lee B, Yanamandra K, Bocchini J. Thiamin deficiency: a possible major cause of some tumors? Oncol Rep. 2005;14:1589–92.
Kabat G, Miller A, Jain M, Rohan T. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816–21.
Acknowledgments
We thank Julia Grimm for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Rights and permissions
About this article
Cite this article
Grimm, M., Calgéer, B., Teriete, P. et al. Targeting thiamine-dependent enzymes for metabolic therapies in oral squamous cell carcinoma?. Clin Transl Oncol 18, 196–205 (2016). https://doi.org/10.1007/s12094-015-1352-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1352-5