Abstract
Purpose
Very-long-chain acyl-CoA dehydrogenase (VLCAD) is an essential mediator in fatty acid metabolism. The progression of human hepatocellular carcinoma (HCC) is closely associated with the disorder of energy supply. Here, we aimed to investigate the role and underlying molecule mechanism of VLCAD in pathological process of HCC.
Methods
In this study, VLCAD was induced silencing and overexpression using small hairpin RNA (shRNA) and lentiviral-mediated vector in HCC cell lines. The proliferation of HCC cells was determined using CCK-8 assay. Transwell assay and lung metastasis were performed to analysis cell metastasis in vitro and in vivo. ECAR and OCR were used to evaluate the activity of glycolysis and mitochondrial oxidative phosphorylation.
Results
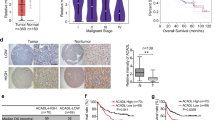
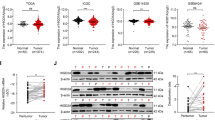
Our data indicated that VLCAD was downregulated in human HCC tissues and cells. VLCAD overexpression strongly suppressed the proliferation and metastasis of HCC cells associating with the decrease of ATP accumulation and glycolysis activity. Importantly, the PI3K/AKT inhibitor LY294002 strongly abolished the role of shVLCAD in HCC cells. Our results suggested that VLCAD suppressed the growth and metastasis in HCC cells by inhibiting the activities of glycolysis and mitochondrial oxidative phosphorylation metabolism via PI3K/AKT pathway.
Conclusions
Together, present findings not only demonstrated the protective role of and molecular network of VLCAD in HCC cells but also indicated its and potential use as a target in the therapy of HCC.






Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:277–300.
Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol. 2015;13:2140–51.
Yeh CS, Wang JY, Cheng TL, Juan CH, Wu CH, Lin SR. Fatty acid metabolism pathway play an important role in carcinogenesis of human colorectal cancers by microarray-bioinformatics analysis. Cancer Lett. 2006;233:297–308.
Monaco ME. Fatty acid metabolism in breast cancer subtypes. Oncotarget. 2017;8:29487–500.
Kanda T, Jiang X, Nakamura M, Haga Y, Sasaki R, Wu S, et al. Overexpression of the androgen receptor in human hepatoma cells and its effect on fatty acid metabolism. Oncol Lett. 2017;13:4481–6.
Cao D, Song X, Che L, Li X, Pilo MG, Vidili G, et al. Both de novo synthetized and exogenous fatty acids support the growth of hepatocellular carcinoma cells. Liver Int. 2016;37:80–9.
Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2015;73:1–16.
Sims HF, Brackett JC, Powell CK, Treem WR, Hale DE, Bennett MJ, et al. The molecular basis of pediatric long chain 3-hydroxyacyl-CoA dehydrogenase deficiency associated with maternal acute fatty liver of pregnancy. Proc Natl Acad Sci USA. 1995;92:841–5.
Tucci S, Primassin S, Veld FT, Spiekerkoetter U. Medium-chain triglycerides impair lipid metabolism and induce hepatic steatosis in very long-chain acyl-CoA dehydrogenase (VLCAD)-deficient mice. Mol Genet Metab. 2010;101:40–7.
Chai R, Fu H, Zheng Z, Liu T, Ji S, Li G. Resveratrol inhibits proliferation and migration through SIRT1 mediated post-translational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol Med Rep. 2017;16:8037–44.
He Y, Liu X. The tumor-suppressor gene LZTS1 suppresses hepatocellular carcinoma proliferation by impairing PI3K/Akt pathway. Biomed Pharmacother. 2015;76:141–6.
Jing-Song C, Xiao-Hui H, Qian W, Xi-Lin C, Xin-Hui F, Hao-Xiang T, et al. FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin Exp Metastasis. 2010;27:71.
Jing-Song C, Xiao-Hui H, Qian W, Jiong-Qiang H, Long-Juan Z, Xi-Lin C, et al. Sonic hedgehog signaling pathway induces cell migration and invasion through focal adhesion kinase/AKT signaling-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9 in liver cancer. Carcinogenesis. 2013;34:10–9.
Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol Ther. 2016;172:101–15.
Michele M, Italia F, Fabiana C, Ursula CI, Anais DC, Nicola I, et al. PTEN: multiple functions in human malignant tumors. Front Oncol. 2015;5:24.
Li Y, Kwan TC, Wang S, Li X, Yang Y, Fu L, et al. MAF1 suppresses AKT-mTOR signaling and liver cancer through activation of PTEN transcription. Hepatology. 2016;63:1928.
Vignarajan S, Xie C, Yao M, Sun Y, Dong Q. Loss of PTEN stabilizes the lipid modifying enzyme cytosolic phospholipase A2α via AKT in prostate cancer cells. Oncotarget. 2014;5:6289–99.
Feng J, Li J, Wu L, et al. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:126.
Protasoni M, Zeviani M. Mitochondrial structure and bioenergetics in normal and disease conditions. Int J Mol Sci. 2021;22:586.
Liberti MV, Locasale JW. The Warburg Effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8.
Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
Zhang X, Guo J, JabbarzadehKaboli P, et al. Analysis of key genes regulating the warburg effect in patients with gastrointestinal cancers and selective inhibition of this metabolic pathway in liver cancer cells. Onco Targets Ther. 2020;13:7295–304.
Huang Q, Huang Q, Chen W, Wang L, Lin W, Lin J, et al. Identification of transgelin as a potential novel biomarker for gastric adenocarcinoma based on proteomics technology. J Cancer Res Clin Oncol. 2008;134:1219–27.
Zhang J, Zhang Q. Using seahorse machine to measure OCR and ECAR in cancer cells. Methods Protoc. 2019.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020.
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9.
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:S0168827818302150.
Acknowledgements
No.
Author information
Authors and Affiliations
Contributions
QZ and XW are responsible for the conception or design of the work. QZ, YY, YZ and XW contribute the acquisition, analysis, or interpretation of data for the work. QZ and YY provide the tissue samples. YZ helps in the follow-up of the patients. XW helps in reviewing the histopathology slides. All authors finally approved the manuscript version to be published. XW is the guarantor of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee of The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University and conducted in accordance with the ethical standards.
Informed consent
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

12094_2021_2733_MOESM1_ESM.jpg
Supplementary file1 Gene set enrichment analysis (GSEA) analysis of VLCAD in HCC tissues. A-B. VLCAD is enrichment in the metastasis and glycolysis of HCC tissues. C-D. VLCAD is negatively correlated with the PI3/AKT pathway in HCC tissues (JPG 274 KB)

12094_2021_2733_MOESM2_ESM.jpg
Supplementary file2 Knockdown and overexpression of VLCAD in HCC cells. A and B. stand for the relative mRNA and protein level of VLCAD in HCC cells (Hep3B, HepG2, Huh7 and SKHEP1) and normal liver cells (LO2). * p < 0.01 vs LO2, ** p < 0.01 vs LO2, *** p < 0.001 vs LO2. C and D. stand for the relative mRNA and protein levels of VLCAD were deeply suppressed in HEP3B and SKHEP1 cells transfected with shVLCAD-1, shVLCAD-2 and shVLCAD-3 respectively. *** p < 0.001 vs shNC. E and F. The level of VLCAD was overexpressed in HepG2 and Huh7 cells transfected with oeVLCAD. *** p < 0.001 vs oeNC (JPG 2728 KB)
Rights and permissions
About this article
Cite this article
Zhu, Q., Yu, Y., Zhang, Y. et al. VLCAD inhibits the proliferation and invasion of hepatocellular cancer cells through regulating PI3K/AKT axis. Clin Transl Oncol 24, 864–874 (2022). https://doi.org/10.1007/s12094-021-02733-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02733-3