Abstract
The preferentially expressed antigen of melanoma (PRAME), a tumor-associated antigen, is considered a prognostic marker for various human malignancies. The prognostic significance of PRAME expression for diffuse large B-cell lymphoma (DLBCL) patients treated with rituximab-containing chemotherapy has not been evaluated to date, and the ability of immunohistochemistry (IHC) to detect PRAME expression in these patients has not yet been studied, although IHC is simple to perform in clinical practice. We evaluated the prognostic significance of PRAME expression based on IHC analysis in 160 DLBCL patients treated with R-CHOP therapy. There was a significant association between higher PRAME expression and shorter progression-free survival (PFS), and a trend toward shorter overall survival (OS) in patients with higher PRAME expression than that in patients with lower PRAME expression (5-year PFS, 48.1 vs. 61.1 %; 5-year OS, 65.6 vs. 79.1 %). Patients with high PRAME expression tended to have lower chemotherapeutic responses. Thus, IHC is useful for detecting and assessing PRAME expression in DLBCL. Further, we found a positive correlation between IHC and quantitative real-time RT-PCR measurements of PRAME expression. Our findings indicate that IHC results of PRAME expression can be a novel prognostic maker in DLBCL patients treated with R-CHOP therapy.
Similar content being viewed by others
Introduction
The preferentially expressed antigen of melanoma (PRAME) was initially isolated as a human melanoma antigen that is recognized by cytotoxic T cells (CTL) [1, 2]. Most normal tissues do not express PRAME, and only a weak expression of PRAME has been observed in the testis, ovaries, adrenal glands, and endometrial cells. In contrast, PRAME is over-expressed in a wide variety of human malignancies such as carcinoma [3], sarcoma [4, 5], and hematologic malignancies [6–8]. Some studies have shown that in various types of solid tumors, PRAME expression is correlated with poor clinical outcome and advanced stage disease [3–5]. It has been reported that in acute and chronic leukemia, PRAME expression increases with disease progression [9–11]. On the other hand, high PRAME expression in childhood acute leukemia was shown to be a marker for a favorable prognosis [12, 13]. Therefore, the clinical significance of PRAME expression is controversial in hematologic malignancies.
Currently, the physiological function of PRAME is not completely understood. Thus far, it has been reported that PRAME acts as a dominant repressor of retinoic acid (RA) receptor signaling and that PRAME inhibits RA-induced cell differentiation and apoptosis [14]. Studies have also shown that the knockdown of PRAME in tumor cell lines can cause a decrease in cell proliferation and increase in apoptosis and cytotoxic drug sensitivity [4, 9, 15].
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma amongst adults, accounting for 30–40 % of non-Hodgkin lymphoma cases. DLBCL is regarded as a heterogeneous disease that presents with a diversity of clinical features and biological characteristics [16]. A number of prognostic markers have been identified in DLBCL patients, including BCL2, BCL6, and cell of origin [17–19]. The introduction of rituximab as part of the chemotherapeutic regimen for patients with lymphoma has markedly improved the prognosis of DLBCL patients [20, 21], and it invalidated prognostic markers that were previously considered to have a significant value [22–24]. Kawano et al. [25] employed reverse transcriptase-polymerase chain reaction (RT-PCR) to examine the prognostic significance of PRAME expression in DLBCL patients, and they found that PRAME expression correlated with shorter progression free survival (PFS) and lower chemotherapeutic responses in DLBCL patients treated with anthracycline-containing chemotherapy. Thus far, the prognostic significance of PRAME expression has not been evaluated in DLBCL patients treated with rituximab-containing chemotherapy. Although it is easy to analyze protein expression in clinical practice using immunohistochemistry (IHC), this method has not been validated for the detection of PRAME expression in DLBCL.
The aim of the present study was to determine the prognostic significance of PRAME expression based on IHC in DLBCL patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) therapy. In addition, we compared PRAME expression as measured by IHC and quantitative real-time RT-PCR (qRT-PCR).
Materials and methods
Patients and tissue samples
We enrolled 160 patients with DLBCL, who were treated with R-CHOP therapy, at the Tokyo Women’s Medical University Hospital in Japan between December 2001 and February 2012. All patients were diagnosed according to the World Health Organization (WHO) criteria. We excluded patients with primary mediastinal large B-cell lymphoma, primary central nervous system lymphoma, and those with DLBCL that transformed from low-grade B-cell lymphoma. We analyzed the following clinical characteristics as recorded at the time of diagnosis: age, gender, performance status (PS), lactate dehydrogenase (LDH) levels, number of extranodal sites, disease stage (according to the Ann Arbor system [26] ), the International prognostic index (IPI) [27], B symptoms, and bulky disease (more than 10 cm). All patients (n = 160) received R-CHOP therapy. Twenty-six patients received involved-field radiotherapy following R-CHOP therapy. Autologous peripheral blood stem cell transplantation (auto-PBSCT) was performed in 13 patients after R-CHOP therapy (one patient received both radiotherapy and auto-PBSCT).
Tissue specimens were obtained at the initial presentation of patients, fixed in formalin, and embedded in paraffin. Cryopreserved tissue samples obtained at the same time were available in 40 patients.
The study was approved by the Ethics Committee of Tokyo Women’s Medical University (approval number: 2550) and conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry
We immunohistochemically stained 4 μm sections of the formalin-fixed, paraffin-embedded tissue samples. The sections were deparaffinized in xylene and rehydrated in graded alcohol. Antigen retrieval was done with target retrieval solution (pH 9; Dako, Glostrup, Denmark) and Pascal pressurized heating chamber (Dako) treatment (125 °C, 40 min). We inhibited endogenous peroxidase activity by incubating the sections in 3 % hydrogen peroxide for 5 min. The inhibition of endogenous peroxidase activity before MYC antibody was omitted in order to retain stable immunostainability. The sections were incubated at room temperature for 60 min with the following primary antibodies: anti-PRAME (1:400, polyclonal, Atlas, Stockholm, Sweden), CD5 (1:50, monoclonal, Dako), CD10 (1:100, monoclonal, Dako), BCL6 (1:10, monoclonal, Dako), MUM-1 (1:400, monoclonal, Abcam, Cambridge, UK), BCL2 (1:50, monoclonal, Dako), and MYC (1:600, monoclonal, Abcam). After incubation with primary antibodies, the sections were washed with phosphate buffered saline and then incubated with dextran coupled with peroxidase molecules and goat secondary antibody molecules against rabbit and mouse immunoglobulins (EnVision, Dako) for 20 min at room temperature. The color was developed with diaminobenzidine tetrahydrochloride chromogen, and the sections were counterstained with hematoxylin. We used 2 score categories (negative or positive) for CD5 expression, as described in the validation study by Salles et al. [28]. Based on the expression of CD10, BCL6, MUM–1, patients were classified as having the germinal center (GC) subtype or non-GC subtype, as defined by the Hans algorithm [29]. A double-hit score (DHS), previously described by Green et al. [30] and based on immunohistochemical MYC and BCL2 expression, was assigned. All sections were evaluated independently by two hematopathologists who were blinded to the clinical outcomes of the patients.
Quantitative real-time reverse transcriptase-polymerase chain reaction
In total, 40 cryopreserved tissue samples from DLBCL patients were analyzed. Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA). One microgram of total RNA was converted to single-stranded cDNA using a random primer and a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocols. We based our measurement of mRNA levels of PRAME on the TaqMan probe method, which utilizes an ABI 7500 real-time system (Applied Biosystems) with co-amplification of the endogenous control gene human β-actin (Applied Biosystems). The real-time amplification reaction was performed in a total volume of 25 μL with a concentration of 300 nM for primers and 200 nM for probes. After adding 2.5 μL of cDNA and 12.5 μL of TaqMan Gene Expression PCR Master Mix (Applied Biosystems), samples were amplified in duplicate wells for each experiment. The relative expression of PRAME was determined by the comparative CT method after normalization with β-actin gene. The human PRAME primer-probe sets were from Applied Biosystems (assay ID: Hs00196132_m1).
Statistical analysis
We considered the following factors to affect the prognosis of DLBCL patients: gender, age, PS, LDH, extranodal sites, disease stage, IPI score, B symptoms, bulky disease, cell of origin, CD5 expression, DHS, and PRAME expression as assessed by IHC. PFS was defined as the interval between the date of initial diagnosis and the date of disease progression or death as a result of any cause [31]. Overall survival (OS) was defined as the interval between the date of initial diagnosis and the date of death as a result of any cause, or the date of last follow-up. Chemotherapy response was assessed after R-CHOP therapy and classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the international workshop criteria [31]. PFS and OS were estimated by means of the Kaplan–Meier method, and a univariate analysis was performed by log-rank test. The hazard ratio was calculated by the Cox proportional hazard model. The association between PRAME expression by IHC and patient characteristics and chemotherapeutic response were compared with Chi square analysis and Mann–Whitney test (age). A t test was used to assess the correlation of PRAME expression between the IHC and qRT-PCR data. After adopting all factors (gender, age, PS, LDH, number of extranodal sites, stage, IPI score, B symptom, bulky disease, cell of origin, CD5 expression, DHS, and PRAME expression) that were used in the univariate analysis, the Cox proportional hazard model was performed as a multivariate analysis. Values of P < 0.05 were considered statistically significant. All statistical analyses were performed using JMP 11 (SAS Institute Japan Ltd.) software.
Results
Patient characteristics
The characteristics of patients are summarized in Table 1. The study cohort enrolled 74 female patients (46.2 %) and 86 male patients (53.8 %). The median age of patients was 66 years (range 17–87 years), and 105 patients (65.6 %) were older than 60 years. Eighty-five patients (53.1 %) had advanced disease (stage III or IV), 26 (16.3 %) had a poor performance status (2–4), 108 (67.5 %) had elevated LDH levels, 67 (41.9 %) had a high-intermediate or high risk (scores of 3, 4, or 5) as defined by IPI, 33 (20.6 %) had B symptoms, and bulky disease was noted in 14 (8.8 %) patients. Only 6 patients (3.8 %) had positive CD5 expression, whilst 106 patients (66.3 %) were classified as having the non-GC subtype. The median follow-up was 2.7 years (range 0.1–10.3 years).
Analysis of PRAME expression by IHC
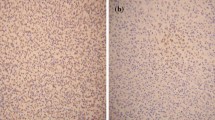
The staining patterns of PRAME expression in tumor cells of DLBCL showed distinct cytoplasmic granules. Stainability varied between tumor cells (Fig. 1). Tumor cells were considered PRAME positive even if they contained only small amounts of stained cytoplasmic granules. As stainability of immunohistochemical PRAME expression in DLBCL is not yet known, we classified all our patients into four categories (0, 1+, 2+, and 3+) based on the percentage of PRAME positive tumor cells that they had (<25 % were classified as 0; 25 % or more but <50 %, as 1+; 50 % or more but <75 %, as 2+; and 75 % or more, as 3+). We did not have any knowledge of patients’ clinical outcome. In total, 109 of the 160 patients (68.1 %) were classified as 0; 17 (10.7 %), as 1+; 13 (8.1 %), as 2+; and 21 (13.1 %), as 3+.
Immunohistochemical staining for PRAME in diffuse large B-cell lymphoma (×400). The tumor cells contain cytoplasmic granules with various levels of stainability. Scoring of PRAME expression was classified into 4 categories based on the percentage of PRAME-positive tumor cells. a, scored as 0 (<25 %); b, scored as 1+ (25 % or more but <50 %); c, scored as 2+ (50 % or more but <75 %); d, scored as 3+ (75 % or more) and high magnification (insert) shows the PRAME-positive tumor cells with cytoplasmic granules (arrow heads) and PRAME-negative tumor cells without cytoplasmic granules (arrow)
The optimal cutoff for PRAME expression by IHC was identified as the value equal to the maximum log-rank statistic that predicts patient survival. Univariate analysis showed that PRAME expression in our group of patients was associated with poor clinical outcome only when a cutoff point of 75 % for positive tumor cell was selected. Therefore, we considered patients classified as 3+ (75 % or more positive tumor cells) to have high PRAME expression (n = 21), and patients classified as 2+ or less (<75 % positive tumor cells) to have low PRAME expression (n = 139) (Table 1).
Kaplan–Meier estimates showed that patients with high PRAME expression had shorter PFS and OS than patients with low PRAME expression (Fig. 2). Furthermore, there was a significant association between a shorter PFS (P = 0.013) and a higher PRAME expression, and a trend toward shorter OS (P = 0.159) in patients with high PRAME expression. The 5-year PFS rate differed significantly between patients with high versus low PRAME expression (48.1 versus 61.1 %, P = 0.013). Similarly, the 5-year OS rate in patients with high PRAME expression was 65.6 %, whereas it was 79.1 % in patients with low PRAME expression (P = 0.130).
Progression-free survival (a) and overall survival (b) according to PRAME expression based on immunohistochemistry. The patients with high PRAME expression (grey) showed significantly poorer progression-free survival (PFS) than the patients with low PRAME expression (black) (P = 0.013). The patients with high PRAME expression (grey) also tended to have a shorter overall survival (OS) than the patients with low PRAME expression (black), but this was not statistically significant (P = 0.159)
Association of PRAME expression with clinicopathologic features and clinical outcomes
There were no significant differences between the clinicopathologic features of patients with high and low PRAME expression (Table 1). We found no significant associations between PRAME expression and disease stage, IPI score (low or high), cell of origin (GC subtype or non-GC subtype), CD5 expression, or DHS. The overall response rate (CR and PR) was 89.9 % in 138 evaluable DLBCL patients. The CR rate was 73.7 % for patients with high PRAME expression and 84.0 % for patients with low PRAME expression (P = 0.269). The PD rate was higher in patients with high PRAME expression (21.1 %) than in those with low PRAME expression (8.4 %), but statistical significance was not reached (P = 0.090).
Table 2 shows the results of univariate analysis. In DLBCL patients, older age, poor PS, elevated LDH, high IPI score (3–5), and high PRAME expression were significant risk factors for PFS, while older age, poor PS, elevated LDH, number of extranodal sites (≥2), advanced disease stage, and high IPI score (3–5) were significant risk factors for OS.
Table 3 shows the results of multivariate analysis. Multivariate analysis found an interaction between B symptoms and PS in prognostic factors; therefore, we identified 4 variants [B symptoms (−) PS 1–2, B symptoms (−) PS 2–4, B symptoms (+) PS 1–2, and B symptoms (+) PS 2–4] for the Cox proportional hazard model. Thus, elevated LDH, high PRAME expression, as well as combined variate of B symptoms (−) PS 2–4, were found to be independent predictors of shorter PFS, while elevated LDH and combined variate of B symptoms (−) PS 2–4 were independent predictors of shorter OS.
Correlation between qRT-PCR and IHC
PRAME expression as measured by qRT-PCR was performed on 40 samples of the enrolled patients. According to our IHC results, 11 of these patients had high and 29 had low PRAME expression (Fig. 3). After examining the correlation, we found that PRAME mRNA expression was significantly higher in patients with high PRAME expression than that in patients with low PRAME expression (P = 0.008).
The relative expression of PRAME mRNA in patients with low and high PRAME expression classified by immunohistochemistry. PRAME mRNA expression was significantly higher in patients with high PRAME expression than that in those with low PRAME expression (P = 0.008). The top and bottom of each diamond represent the 95 % confidence interval for each group. The mean line across the middle of each diamond represents the group mean. Overlap marks appear as lines above and below the group mean
Discussion
In this study, we confirmed that immunohistochemical analysis is a useful technique for the detection and assessment of PRAME expression in DLBCL. Univariate and multivariate analysis showed that high PRAME expression was significantly associated with shorter PFS in DLBCL patients treated with R-CHOP therapy, suggesting that high PRAME expression, based on IHC, is a useful marker for predicting poor prognosis in DLBCL patients treated with rituximab-containing chemotherapy. On the other hand, IPI score, which is often used in prognostic models to predict the outcome of patients with DLBCL, did not predict shorter PFS or shorter OS in our multivariate analysis. The relatively small number of cases enrolled in the present study could have affected this result.
In addition, we found a positive correlation between PRAME expression as classified by IHC and PRAME expression as measured by qRT-PCR.
In various malignancies, an association between high PRAME expression and a poor prognosis and advanced stage disease has previously been reported. For instance, high PRAME expression was found in advanced stage of neuroblastoma, and it was found to be associated with shorter event-free survival in that study [5]. High PRAME expression was an independent marker of short metastasis-free intervals in patients with breast cancer [3] and was found to be associated with poor overall survival and lung metastases in patients with osteosarcoma [4].
In hematologic malignancies, the clinical significance of PRAME expression is controversial. Tanaka et al. [9] found PRAME expression in acute leukemia to be higher during relapse than at the time of diagnosis, and in chronic myeloid leukemia, PRAME expression was shown to increase with disease progression from the chronic to the advanced phase [10, 11]. On the other hand, high PRAME expression was shown to be correlated with a good prognosis in acute myeloid leukemia (AML) in both adult [32] and pediatric patients [12], as well as in childhood lymphoblastic leukemia [13].
Kawano et al. [25] showed, by means of cDNA microarray analysis, that the expression of the PRAME gene is markedly increased in DLBCL patients resistant to anthracycline-containing chemotherapy. They also found that DLBCL patients with PRAME expression, as detected by RT-PCR, had a shorter PFS and lower response to anthracycline-containing chemotherapy than DLBCL patients without PRAME expression. Similarly, we found that there was an association between high PRAME expression and poor PFS and a trend toward low chemotherapeutic responses in DLBCL patients treated with R-CHOP therapy, which is currently the standard treatment for DLBCL. We did not find a statistically significant difference in the OS and response rates of patients with high or low PRAME expression, but our results might have been affected by our methodology (the use of IHC rather than RT-PCR) or the relatively small number of cases enrolled in our study.
Although the function of PRAME has not yet been fully elucidated, it is known that PRAME has putative nuclear receptor (NR) boxes, which suggests that it serves as a transcription regulator of nuclear receptor signaling. Epping et al. [14] illustrated that in the presence of RA, PRAME interacts with the RA receptor (RAR) via NR boxes, and this, in turn, prevents ligand-induced receptor activation and target gene transcription. PRAME inhibits RA-induced cell differentiation, growth arrest, and apoptosis, as it acts as a dominant repressor of RAR. In addition, PRAME knockdown was shown to decrease the proliferation of melanoma cells as well as other solid cancer cells [4, 14]. PRAME overexpression also has clinical implications in leukemia. We previously reported that PRAME knockdown caused a decrease in the colony formation and growth rate, as well as G0/G1-phase cell cycle arrest in K562 cells, which are known to highly express PRAME. This suggests that PRAME expression has a role in the progression of acute leukemia [9]. Furthermore, Bullinger et al. found that PRAME impaired differentiation and increased proliferation of leukemia cells because it inhibited RAR signaling in AML without RAR rearrangement [33]. Kewitz et al. also examined the effect of PRAME knockdown on Hodgkin lymphoma cell lines. Interestingly, in their studies, Kewitz et al. [15] found that PRAME knockdown resulted in the restoration of RAR signaling, but increased the sensitivity of Hodgkin lymphoma cells to cytotoxic agents.
Taken together, although the clinical implications of PRAME overexpression in DLBCL cells have not yet been clarified, our findings suggest that lymphoma cells with high PRAME expression acquire growth and survival advantages and resistance to chemotherapeutic agents, which result in low chemotherapeutic response and poor survival rates. Moreover, our results suggest that R-CHOP therapy is not an adequate therapeutic regimen for DLBCL patients with high PRAME expression, and adding a molecular target for PRAME to the conventional chemotherapeutic regimen may improve the clinical outcomes of these patients.
In conclusion, to our knowledge, the present study is the first to show the prognostic significance of PRAME expression in DLBCL patients treated with R-CHOP therapy. PRAME expression based on IHC is a novel maker of a poor prognosis in DLBCL patients treated with rituximab-containing standard chemotherapy. Further studies, including large prospective studies, are necessary to confirm the prognostic significance of PRAME expression, and to clarify the mechanism that leads to the poor prognosis in DLBCL patients with high PRAME expression.
References
Ikeda H, Lethé B, Lehmann F, Van Baren N, Baurain JF, de Smet C, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208.
van Baren N, Chambost H, Ferrant A, Michaux L, Ikeda H, Millard I, et al. PRAME, a gene encoding an antigen recognized on a human melanoma by cytolytic T cells, is expressed in acute leukaemia cells. Br J Haematol. 1998;102:1376–9.
Epping MT, Hart AA, Glas AM, Krijgsman O, Bernards R. PRAME expression and clinical outcome of breast cancer. Br J Cancer. 2008;99:398–403.
Tan P, Zou C, Yong B, Han J, Zhang L, Su Q, et al. Expression and prognostic relevance of PRAME in primary osteosarcoma. Biochem Biophys Res Commun. 2012;419:801–8.
Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M. The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2004;10:4307–13.
Proto-Siqueira R, Falcão RP, de Souza CA, Ismael SJ, Zago MA. The expression of PRAME in chronic lymphoproliferative disorders. Leuk Res. 2003;27:393–6.
Paydas S, Tanriverdi K, Yavuz S, Seydaoglu G. PRAME mRNA levels in cases with chronic leukemia: clinical importance and review of the literature. Leuk Res. 2007;31:365–9.
Paydas S, Tanriverdi K, Yavuz S, Disel U, Baslamisli F, Burgut R. PRAME mRNA levels in cases with acute leukemia: clinical importance and future prospects. Am J Hematol. 2005;79:257–61.
Tanaka N, Wang YH, Shiseki M, Takanashi M, Motoji T. Inhibition of PRAME expression causes cell cycle arrest and apoptosis in leukemic cells. Leuk Res. 2011;35:1219–25.
Watari K, Tojo A, Nagamura-Inoue T, Nagamura F, Takeshita A, Fukushima T, et al. Identification of a melanoma antigen, PRAME, as a BCR/ABL-inducible gene. FEBS Lett. 2000;466:367–71.
Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci. 2006;103:2794–9.
Steinbach D, Hermann J, Viehmann S, Zintl F, Gruhn B. Clinical implications of PRAME gene expression in childhood acute myeloid leukemia. Cancer Genet Cytogenet. 2002;133:118–23.
Steinbach D, Viehmann S, Zintl F, Gruhn B. PRAME gene expression in childhood acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2002;138:89–91.
Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–47.
Kewitz S, Staege MS. Knock-down of PRAME increases retinoic acid signaling and cytotoxic drug sensitivity of Hodgkin lymphoma cells. PLoS One. 2013;8:e55897.
Lossos IS. Molecular pathogenesis of diffuse large B-cell lymphoma. J Clin Oncol. 2005;23:6351–7.
Hermine O, Haioun C, Lepage E, d’Agay MF, Briere J, Lavignac C, et al. Prognostic significance of bcl-2 protein expression in aggressive non-Hodgkin’s lymphoma. Groupe d’Etude des Lymphomes de l’Adulte (GELA). Blood. 1996;87:265–72.
Lossos IS, Jones CD, Warnke R, Natkunam Y, Kaizer H, Zehnder JL, et al. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001;98:945–51.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11.
Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera international trial (MInT) Group. Lancet Oncol. 2006;7:379–91.
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5.
Mounier N, Briere J, Gisselbrecht C, Emile JF, Lederlin P, Sebban C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2–associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood. 2003;101:4279–84.
Winter JN, Weller EA, Horning SJ, Krajewska M, Variakojis D, Habermann TM, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107:4207–13.
Nyman H, Adde M, Karjalainen-Lindsberg ML, Taskinen M, Berglund M, Amini RM, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007;109:4930–5.
Kawano R, Karube K, Kikuchi M, Takeshita M, Tamura K, Uike N, et al. Oncogene associated cDNA microarray analysis shows PRAME gene expression is a marker for response to anthracycline containing chemotherapy in patients with diffuse large B-cell lymphoma. J Clin Exp Hematop. 2009;49:1–7.
Moormeier JA, Williams SF, Golomb HM. The staging of non-Hodgkin’s lymphomas. Semin Oncol. 1990;17:43–50.
A predictive model for aggressive non-Hodgkin’s lymphoma. the International non-Hodgkin’s lymphoma prognostic factors project. N Engl J Med. 1993;329:987–94.
Salles G, de Jong D, Xie W, Rosenwald A, Chhanabhai M, Gaulard P, et al. Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: a study from the Lunenburg lymphoma biomarker consortium. Blood. 2011;117:7070–8.
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82.
Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–7.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Greiner J, Schmitt M, Li L, Giannopoulos K, Bosch K, Schmitt A, et al. Expression of tumor-associated antigens in acute myeloid leukemia: implications for specific immunotherapeutic approaches. Blood. 2006;108:4109–17.
Bullinger L, Schlenk RF, Götz M, Botzenhardt U, Hofmann S, Russ AC, et al. PRAME-induced inhibition of retinoic acid receptor signaling-mediated differentiation–a possible target for ATRA response in AML without t(15;17). Clin Cancer Res. 2013;19:2562–71.
Acknowledgments
We would like to thank all the physicians who were involved in the expert care of the patients during the study period. We also thank the laboratory technicians of the Department of Hematology and Surgical Pathology for excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mitsuhashi, K., Masuda, A., Wang, YH. et al. Prognostic significance of PRAME expression based on immunohistochemistry for diffuse large B-cell lymphoma patients treated with R-CHOP therapy. Int J Hematol 100, 88–95 (2014). https://doi.org/10.1007/s12185-014-1593-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1593-z