Abstract
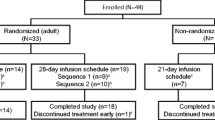
Studies investigating the safety of IgPro10 (Privigen®, CSL Behring, King of Prussia, PA, USA) in Japanese patients with primary immunodeficiency (PID) are lacking. This study evaluated safety and tolerability of IgPro10 in Japanese patients with PID. In this prospective, open-label, single-arm, registrational study for Japan, IgPro10 was administered intravenously at pre-study doses of 138–556 mg/kg body weight per 3-/4-weekly dosing cycle for up to 4 months. Frequency and intensity of adverse events (AEs), their relationship to IgPro10 and AE rate per infusion (AERI) were evaluated. Of 11 enrolled patients, 10 completed the study. The median (range) total duration of exposure was 16.14 (4.1–16.3) weeks. Eight patients reported 19 AEs, none severe (based on maximum severity), giving an AERI of 0.442. One AE was deemed related to IgPro10 treatment. Three patients experienced temporally associated AEs. No serious AEs or deaths were reported. Nine patients (90%) who completed the study tolerated flow rates of ≥ 8 mg/kg/min; 5 tolerated 12 mg/kg/min (7.2 mL/kg/h), translating into a threefold decrease in mean infusion time. IgPro10 was well tolerated at a flow rate of up to 12 mg/kg/min. Safety and tolerability findings were consistent with previously reported studies in non-Japanese patients with PID.

Similar content being viewed by others
References
Ballow M, Notarangelo L, Grimbacher B, Cunningham-Rundles C, Stein M, Helbert M, et al. Immunodeficiencies. Clin Exp Immunol. 2009;158(Suppl 1):14–22.
Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35:696–726.
Wood P, Stanworth S, Burton J, Jones A, Peckham DG, Green T, et al. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol. 2007;149:410–23.
Gardulf A, Nicolay U, Math D, Asensio O, Bernatowska E, Bock A, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114:936–42.
Bousfiha A, Jeddane L, Al-Herz W, Ailal F, Casanova JL, Chatila T, et al. The 2015 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2015;35:727–38.
Ishimura M, Takada H, Doi T, Imai K, Sasahara Y, Kanegane H, et al. Nationwide survey of patients with primary immunodeficiency diseases in Japan. J Clin Immunol. 2011;31:968–76.
Kearns S, Kristofek L, Bolgar W, Seidu L, Kile S. Clinical profile, dosing, and quality-of-life outcomes in primary immune deficiency patients treated at home with immunoglobulin G: data from the IDEaL Patient Registry. J Manag Care Spec Pharm. 2017;23:400–6.
Jolles S, Orange JS, Gardulf A, Stein MR, Shapiro R, Borte M, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179:146–60.
Wasserman RL, Melamed I, Nelson RP Jr, Knutsen AP, Fasano MB, Stein MR, et al. Pharmacokinetics of subcutaneous IgPro20 in patients with primary immunodeficiency. Clin Pharmacokinet. 2011;50:405–14.
Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100.
Berger M. Choices in IgG replacement therapy for primary immune deficiency diseases: subcutaneous IgG vs. intravenous IgG and selecting an optimal dose. Curr Opin Allergy Clin Immunol. 2011;11:532–8.
Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28:413–37.
Kanegane H, Imai K, Yamada M, Takada H, Ariga T, Bexon M, et al. Efficacy and safety of IgPro20, a subcutaneous immunoglobulin, in Japanese patients with primary immunodeficiency diseases. J Clin Immunol. 2014;34:204–11.
Shapiro RS, Wasserman RL, Bonagura V, Gupta S. Emerging paradigm of primary immunodeficiency disease: individualizing immunoglobulin dose and delivery to enhance outcomes. J Clin Immunol. 2017;37:190–6.
Bonagura VR. Using intravenous immunoglobulin (IVIG) to treat patients with primary immune deficiency disease. J Clin Immunol. 2013;33(Suppl 2):S90–4.
Wasserman RL, Manning SC. Diagnosis and treatment of primary immunodeficiency disease: the role of the otolaryngologist. Am J Otolaryngol. 2011;32:329–37.
Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M, Subcutaneous Ig GSG. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–73.
Church JA, Leibl H, Stein MR, Melamed IR, Rubinstein A, Schneider LC, et al. Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin [IGIV 10%] in patients with primary immunodeficiency. J Clin Immunol. 2006;26:388–95.
Stein MR, Nelson RP, Church JA, Wasserman RL, Borte M, Vermylen C, et al. Safety and efficacy of Privigen, a novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29:137–44.
CSL Behring. Privigen (Immune Globulin Intravenous [Human] 10% liquid) package insert. Bern, Switzerland. 2020. https://www.privigen.com/prescribing-information. Accessed Nov 2020.
Church JA, Borte M, Taki H, Nelson RP, Sleasman JW, Knutsen AP, et al. Efficacy and safety of privigen in children and adolescents with primary immunodeficiency. Pediatr Asthma Allergy Immunol. 2009;22:53–61.
Sleasman JW, Duff CM, Dunaway T, Rojavin MA, Stein MR. Tolerability of a new 10% liquid immunoglobulin for intravenous use, Privigen, at different infusion rates. J Clin Immunol. 2010;30:442–8.
Dorsey MJ, Ho V, Mabudian M, Soler-Palacin P, Dominguez-Pinilla N, Rishi R, et al. Clinical experience with an L-proline-stabilized 10% intravenous immunoglobulin (Privigen(R)): real-life effectiveness and tolerability. J Clin Immunol. 2014;34:804–12.
Morio T, Baheti G, Tortorici MA, Hofmann J, Rojavin MA. Pharmacokinetic properties of Privigen® in Japanese patients with primary immunodeficiency. Immunol Med. 2019;42:162–8.
Luo D, Baheti G, Tortorici MA, Hofmann J, Rojavin MA. Pharmacometric analysis of IgPro10 in Japanese and non-Japanese patients with primary immunodeficiency. Clin Ther. 2020;42:196–209.
Jolles S, Bernatowska E, de Gracia J, Borte M, Cristea V, Peter HH, et al. Efficacy and safety of Hizentra(®) in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol. 2011;141:90–102.
Hagan JB, Fasano MB, Spector S, Wasserman RL, Melamed I, Rojavin MA, et al. Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency. J Clin Immunol. 2010;30:734–45.
Wasserman RL, Melamed I, Kobrynski L, Strausbaugh SD, Stein MR, Sharkhawy M, et al. Efficacy, safety, and pharmacokinetics of a 10% liquid immune globulin preparation (GAMMAGARD LIQUID, 10%) administered subcutaneously in subjects with primary immunodeficiency disease. J Clin Immunol. 2011;31:323–31.
Berger M, Cunningham-Rundles C, Bonilla FA, Melamed I, Bichler J, Zenker O, et al. Carimune NF Liquid is a safe and effective immunoglobulin replacement therapy in patients with primary immunodeficiency diseases. J Clin Immunol. 2007;27:503–9.
Stein MR. The new generation of liquid intravenous immunoglobulin formulations in patient care: a comparison of intravenous immunoglobulins. Postgrad Med. 2010;122:176–84.
Bjorkander J, Nikoskelainen J, Leibl H, Lanbeck P, Wallvik J, Lumio JT, et al. Prospective open-label study of pharmacokinetics, efficacy and safety of a new 10% liquid intravenous immunoglobulin in patients with hypo- or agammaglobulinemia. Vox Sang. 2006;90:286–93.
Roifman CM, Schroeder H, Berger M, Sorensen R, Ballow M, Buckley RH, et al. Comparison of the efficacy of IGIV-C, 10% (caprylate/chromatography) and IGIV-SD, 10% as replacement therapy in primary immune deficiency. A randomized double-blind trial. Int Immunopharmacol. 2003;3:1325–33.
Frenzel W, Wietek S, Svae TE, Debes A, Svorc D. Tolerability and safety of Octagam(R) (IVIG): a post-authorization safety analysis of four non-interventional phase IV trials. Int J Clin Pharmacol Ther. 2016;54:847–55.
Berger M, Pinciaro PJ, Flebogamma I. Safety, efficacy, and pharmacokinetics of Flebogamma 5% [immune globulin intravenous (human)] for replacement therapy in primary immunodeficiency diseases. J Clin Immunol. 2004;24:389–96.
Acknowledgements
Editorial and graphical support was provided by Heather Shawcross, PhD, CMPP™ of Fishawack Communications GmbH, Basel, Switzerland, a member of Fishawack Health, supported by CSL Behring. The data summarized in this study are from a CSL Behring-sponsored clinical trial.
Funding
The authors declare that this study received funding from CSL Behring. The funder had the following involvement with the study: study design, data collection and analysis, and decision to publish.
Author information
Authors and Affiliations
Contributions
TM, KG, TI, KM, HO, KY, JH, JPL, AS and MAR have made substantial contributions to the conceptualization, investigation, methodology, validation of results, visualization, review and editing of the manuscript. All the authors have approved the version to be published and are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
ASH, MAR, JH and JPL are employees of CSL Behring. MAR, ASH, JH and JPL own CSL Behring shares. TM has received grant from CSL Behring. KG, TI, KM, HO, KY have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Morio, T., Gotoh, K., Imagawa, T. et al. Safety and tolerability of IgPro10 in Japanese primary immunodeficiency patients: a registrational study. Int J Hematol 113, 921–929 (2021). https://doi.org/10.1007/s12185-021-03106-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03106-w