Abstract
Background
Idiopathic nephrotic syndrome is a common form of glomerular nephropathy in children, with an incidence rate of 1.15–16.9/100,000 depending on different nationalities and ethnicities. The etiological factors and mechanisms of childhood idiopathic nephrotic syndrome have not yet been fully elucidated. This review summarizes the progress of the immunopathogenesis of idiopathic nephrotic syndrome in children.
Data sources
We review the literature on the immunopathogenesis of idiopathic nephrotic syndrome in children. Databases including Medline, Scopus, and Web of Science were searched for studies published in any language with the terms “children”, “idiopathic nephrotic syndrome”, “immunopathogenesis”, “T cells”, “circulating permeability factors”, and “B cells”.
Results
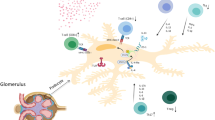
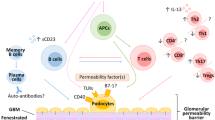
Dysfunction in T lymphocytes and pathogenic circulatory factors were indicated to play key roles in the pathogenesis of idiopathic nephrotic syndrome. Recently, some studies have shown that cellular immune dysfunction may also be involved in the pathogenesis of idiopathic nephrotic syndrome.
Conclusions
Both T- and B-cell dysfunction may play significant roles in the pathogenesis of idiopathic nephrotic syndrome, like two sides of one coin, but the role of B cell seems more important than T cells.

Similar content being viewed by others
References
Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines–application to the individual patient. Kidney Int. 2012;82:840–56.
Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018;392:61–74.
Roth KS, Amaker BH, Chan JC. Nephrotic syndrome: pathogenesis and management. Pediatr Rev. 2002;23:237–48.
Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62:403–41.
Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–9.
Martinelli R, Okumura AS, Pereira LJ, Rocha H. Primary focal segmental glomerulosclerosis in children: prognostic factors. Pediatr Nephrol. 2001;16:658–61.
Paik KH, Lee BH, Cho HY, Kang HG, Ha IS, Cheong HI, et al. Primary focal segmental glomerular sclerosis in children: clinical course and prognosis. Pediatr Nephrol. 2007;22:389–95.
Trautmann A, Schnaidt S, Lipska-Zietkiewicz BS, Bodria M, Ozaltin F, Emma F, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055–65.
Davin JC. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr Nephrol. 2016;31:207–15.
Daniel V, Trautmann Y, Konrad M, Nayir A, Scharer K. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin Nephrol. 1997;47:289–97.
Kaneko K, Tsuji S, Kimata T, Kitao T, Yamanouchi S, Kato S. Pathogenesis of childhood idiopathic nephrotic syndrome: a paradigm shift from T cells to podocytes. World J Pediatr. 2015;11:21–8.
Ishikura K, Ikeda M, Hattori S, Yoshikawa N, Sasaki S, Iijima K, et al. Effective and safe treatment with cyclosporine in nephrotic children: a prospective, randomized multicenter trial. Kidney Int. 2008;73:1167–73.
Lin CY, Hsu HC. Histopathological and immunological studies in spontaneous remission of nephrotic syndrome after intercurrent measles infection. Nephron. 1986;42:110–5.
Cunard R, Kelly CJ. T cells and minimal change disease. J Am Soc Nephrol. 2002;13:1409–11.
Federico A, Merletti MG, Lisi E, De Finis F, Trivelli G, Sopranzi F. Nephrotic syndrome as first presentation of malignant thymoma: description of a clinical case. G Ital Nefrol. 2010;27:674–9.
Geylis M, Rosen GB, Danino D, Schreiber R, Hassan D, Nalbandyan K, et al. Hodgkin’s lymphoma, nephrotic syndrome, and echinococcosis cysts: an unusual association and literature review. Pediatr Hematol Oncol. 2019;36:40–5.
Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–7.
Cara-Fuentes G, Wasserfall CH, Wang H, Johnson RJ, Garin EH. Minimal change disease: a dysregulation of the podocyte CD80-CTLA-4 axis? Pediatr Nephrol. 2014;29:2333–40.
Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, et al. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20:260–6.
Ling C, Liu X, Shen Y, Chen Z, Fan J, Jiang Y, et al. Urinary CD80 levels as a diagnostic biomarker of minimal change disease. Pediatr Nephrol. 2015;30:309–16.
Ahmed HM, Ezzat DA, Doudar NA, Adel M. Urinary CD80 as a replacement for renal biopsy for diagnosis of pediatric minimal change disease. Iran J Kidney Dis. 2018;12:107–11.
Tsuji S, Kimata T, Yamanouchi S, Kitao T, Kino J, Suruda C, et al. Regulatory T cells and CTLA-4 in idiopathic nephrotic syndrome. Pediatr Int. 2017;59:643–6.
Zhao B, Han H, Zhen J, Yang X, Shang J, Xu L, et al. CD80 and CTLA-4 as diagnostic and prognostic markers in adult-onset minimal change disease: a retrospective study. PeerJ. 2018;6:e5400.
Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416–23.
Benigni A, Gagliardini E, Remuzzi G. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1261–3.
Alachkar N, Carter-Monroe N, Reiser J. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1263–4.
Kaneko K, Tsuji S, Kimata T. Role of gut microbiota in idiopathic nephrotic syndrome in children. Med Hypotheses. 2017;108:35–7.
Hoyer JR, Vernier RL, Najarian JS, Raij L, Simmons RL, Michael AF. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet. 1972;2:343–8.
McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115–21.
Ali AA, Wilson E, Moorhead JF, Amlot P, Abdulla A, Fernando ON, et al. Minimal-change glomerular nephritis. Normal kidneys in an abnormal environment? Transplantation. 1994;58:849–52.
Le Berre L, Godfrin Y, Gunther E, Buzelin F, Perretto S, Smit H, et al. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J Clin Invest. 2002;109:491–8.
Haffner K, Zimmerhackl LB, von Schnakenburg C, Brandis M, Pohl M. Complete remission of post-transplant FSGS recurrence by long-term plasmapheresis. Pediatr Nephrol. 2005;20:994–7.
Kemper MJ, Wolf G, Muller-Wiefel DE. Transmission of glomerular permeability factor from a mother to her child. N Engl J Med. 2001;344:386–7.
Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34:171–354.
Kashgary A, Sontrop JM, Li L, Al-Jaishi AA, Habibullah ZN, Alsolaimani R, et al. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: A systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 2016;17:104.
Cheung PK, Stulp B, Immenschuh S, Borghuis T, Baller JF, Bakker WW. Is 100KF an isoform of hemopexin? Immunochemical characterization of the vasoactive plasma factor 100KF. J Am Soc Nephrol. 1999;10:1700–8.
Lennon R, Singh A, Welsh GI, Coward RJ, Satchell S, Ni L, et al. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol. 2008;19:2140–9.
Bakker WW, van Dael CM, Pierik LJ, van Wijk JA, Nauta J, Borghuis T, et al. Altered activity of plasma hemopexin in patients with minimal change disease in relapse. Pediatr Nephrol. 2005;20:1410–5.
Savin VJ, Sharma M, Zhou J, Gennochi D, Fields T, Sharma R, et al. Renal and hematological effects of CLCF-1, a B-cell-stimulating cytokine of the IL-6 family. J Immunol Res. 2015;2015:714964.
Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63.
Peng Z, Mao J, Chen X, Cai F, Gu W, Fu H, et al. Serum suPAR levels help differentiate steroid resistance from steroid-sensitive nephrotic syndrome in children. Pediatr Nephrol. 2015;30:301–7.
Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–60.
Kronbichler A, Saleem MA, Meijers B, Shin JI. Soluble urokinase receptors in focal segmental glomerulosclerosis: a review on the scientific point of view. J Immunol Res. 2016;2016:2068691.
Meijers B, Maas RJ, Sprangers B, Claes K, Poesen R, Bammens B, et al. The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis. Kidney Int. 2014;85:636–40.
Sinha A, Bajpai J, Saini S, Bhatia D, Gupta A, Puraswani M, et al. Serum-soluble urokinase receptor levels do not distinguish focal segmental glomerulosclerosis from other causes of nephrotic syndrome in children. Kidney Int. 2014;85:649–58.
Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–32.
Keisuke S, Kohei M, Takuji E, Tomoki M, Yuichi M, Rina O, et al. Role of cathepsin L in idiopathic nephrotic syndrome in children. Med Hypotheses. 2020;141:109718.
Chugh SS, Clement LC, Mace C. New insights into human minimal change disease: lessons from animal models. Am J Kidney Dis. 2012;59:284–92.
Clement LC, Avila-Casado C, Mace C, Soria E, Bakker WW, Kersten S, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–22.
Clement LC, Mace C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46.
Cara-Fuentes G, Segarra A, Silva-Sanchez C, Wang H, Lanaspa MA, Johnson RJ, et al. Angiopoietin-like-4 and minimal change disease. PLoS ONE. 2017;12:e0176198.
Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22:2098–105.
Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D’Agati VD, et al. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol. 2015;26:1443–8.
Clark AJ, Jabs K, Hunley TE, Jones DP, VanDeVoorde RG, Anderson C, et al. Urinary apolipoprotein AI in children with kidney disease. Pediatr Nephrol. 2019;34:2351–60.
Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46.
Ahmad A, Mitrofanova A, Bielawski J, Yang Y, Marples B, Fornoni A, et al. Sphingomyelinase-like phosphodiesterase 3b mediates radiation-induced damage of renal podocytes. FASEB J. 2017;31:771–80.
LaConte L, Mukherjee K. Structural constraints and functional divergences in CASK evolution. Biochem Soc Trans. 2013;41:1017–22.
Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol. 2004;165:923–36.
Caruana G. Genetic studies define MAGUK proteins as regulators of epithelial cell polarity. Int J Dev Biol. 2002;46:511–8.
Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteom. 2005;4:1920–32.
Beaudreuil S, Zhang X, Herr F, Harper F, Candelier JJ, Fan Y, et al. Circulating CASK is associated with recurrent focal segmental glomerulosclerosis after transplantation. PLoS ONE. 2019;14:e0219353.
Ling C, Wang X, Chen Z, Fan J, Meng Q, Zhou N, et al. Altered B-lymphocyte homeostasis in idiopathic nephrotic syndrome. Front Pediatr. 2019;7:377.
Printza N, Papachristou F, Tzimouli V, Taparkou A, Kanakoudi-Tsakalidou F. Peripheral CD19+ B cells are increased in children with active steroid-sensitive nephrotic syndrome. NDT Plus. 2009;2:435–6.
Hsiao CC, Tu KH, Hsieh CY, Lee CC, Chang CH, Fan PC, et al. Immunoglobulin E and G levels in predicting minimal change disease before renal biopsy. Biomed Res Int. 2018;2018:3480309.
Audard V, Larousserie F, Grimbert P, Abtahi M, Sotto JJ, Delmer A, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–60.
Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002;29:2–9.
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384:1273–81.
Basu B, Sander A, Roy B, Preussler S, Barua S, Mahapatra TKS, et al. Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: a randomized clinical trial. JAMA Pediatr. 2018;172:757–64.
Ravani P, Bonanni A, Ghiggeri GM. Randomised controlled trial comparing ofatumumab to rituximab in children with steroid-dependent and calcineurin inhibitor-dependent idiopathic nephrotic syndrome: study protocol. BMJ Open. 2017;7:e013319.
Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med. 2014;370:1268–70.
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21.
Ahmad SB, Appel GB. Antigens, antibodies, and membranous nephropathy: a decade of progress. Kidney Int. 2020;97:29–31.
De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–30.
Godel M, Grahammer F, Huber TB. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2015;372:1073.
Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–74.
van de Logt AE, Fresquet M, Wetzels JF, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96:1292–302.
Funding
This study was supported by the National Natural Foundation of China (81770710), Key Research and Development Plan of Zhejiang Province (2019C03028), the Major projects jointly constructed by the Zhejiang Province, and National Health Commission (WKJ-ZJ-1908).
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation of this review and approved the text.
Corresponding author
Ethics declarations
Ethical approval
Not required for the review.
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Qiao, XH. & Mao, JH. Immunopathogenesis of idiopathic nephrotic syndrome in children: two sides of the coin. World J Pediatr 17, 115–122 (2021). https://doi.org/10.1007/s12519-020-00400-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-020-00400-1