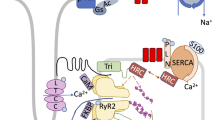
Abstract
Normal heart contraction and rhythm relies on the proper flow of calcium ions (Ca2+) into cardiac cells and between their intracellular organelles, and any disruption can lead to arrhythmia and sudden cardiac death. Electrical excitation of the surface membrane activates voltage-dependent L-type Ca2+ channels to open and allow Ca2+ to enter the cytoplasm. The subsequent increase in cytoplasmic Ca2+ concentration activates calcium release channels (RyR2) located at specialised Ca2+ release sites in the sarcoplasmic reticulum (SR), which serves as an intracellular Ca2+ store. Animal models have provided valuable insights into how intracellular Ca2+ transport mechanisms are altered in human heart failure. The aim of this review is to examine how Ca2+ release sites are remodelled in heart failure and how this affects intracellular Ca2+ transport with an emphasis on Ca2+ release mechanisms in the SR. Current knowledge on how heart failure alters the regulation of RyR2 by Ca2+ and Mg2+ and how these mechanisms control the activity of RyR2 in the confines of the Ca2+ release sites is reviewed.
Similar content being viewed by others
References
Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM (2005) Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97:1314–1322
Asghari P, Schulson M, Scriven DR, Martens G, Moore ED (2009) Axial tubules of rat ventricular myocytes form multiple junctions with the sarcoplasmic reticulum. Biophys J 96:4651–4660. doi:10.1016/j.bpj.2009.02.058
Asghari P, Scriven DR, Sanatani S, Gandhi SK, Campbell AI, Moore ED (2014) Nonuniform and variable arrangements of ryanodine receptors within mammalian ventricular couplons. Circ Res 115:252–262. doi:10.1161/CIRCRESAHA.115.303897
Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C (2009) Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci USA 106:22275–22280. doi:10.1073/pnas.0908971106
Belevych AE, Terentyev D, Terentyeva R et al (2011) The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res 90:493–502. doi:10.1093/cvr/cvr025
Bers DM (2001) Excitation-contraction coupling and cardiac contractile force, 2nd edn. Kluwer Academic Publications, Dordrecht
Bers DM (2002a) Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res 90:14–17
Bers DM (2002b) Cardiac excitation-contraction coupling. Nature 415:198–205
Bers DM (2006) Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21:380–387
Bers DM, Stiffel VM (1993) Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol 264:C1587–C1593
Bers DM, Eisner DA, Valdivia HH (2003) Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res 93:487–490
Braunwald E, Chidsey CA (1965) The adrenergic nervous system in the control of the normal and failing heart. Proc R Soc Med 58:1063–1066
Bristow MR, Minobe W, Rasmussen R et al (1982) Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307:205–211. doi:10.1056/NEJM198207223070401
Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H (2005) Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci USA 102:3099–3104. doi:10.1073/pnas.0500059102
Cannell MB, Kong CH, Imtiaz MS, Laver DR (2013) Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys J 104:2149–2159. doi:10.1016/j.bpj.2013.03.058
Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S (2002) Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem 238:163–179
Chen SR, Li P, Zhao M, Li X, Zhang L (2002) Role of the proposed pore-forming segment of the Ca2+ release channel (ryanodine receptor) in ryanodine interaction. Biophys J 82:2436–2447
Chen W, Wang R, Chen B et al (2014) The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+−triggered arrhythmias. Nat Med 20:184–192. doi:10.1038/nm.3440
Ching LL, Williams AJ, Sitsapesan R (2000) Evidence for Ca 2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res 87:201–206
Cohn JN, Levine TB, Olivari MT et al (1984) Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311:819–823. doi:10.1056/NEJM198409273111303
Crossman DJ, Ruygrok PR, Soeller C, Cannell MB (2010) Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One 6:e17901. doi:10.1371/journal.pone.0017901
Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG (2001) Gender influences on sarcoplasmic reticulum Ca2+ handling in failing human myocardium. J Mol Cell Cardiol 33:1345–1353. doi:10.1006/jmcc.2001.1394
Dulhunty AF, Beard NA, Pouliquin P, Casarotto MG (2007) Agonists and antagonists of the cardiac ryanodine receptor: potential therapeutic agents? Pharmacol Ther 113:247–263
Eisner DA, Trafford AW (2002) Heart failure and the ryanodine receptor: does Occam’s razor rule? Circ Res 91:979–981
Eisner DA, Trafford AW, Diaz ME, Overend CL, O’Neill SC (1998) The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res 38:589–604
Frank KF, Bolck B, Brixius K, Kranias EG, Schwinger RH (2002) Modulation of SERCA: implications for the failing human heart. Basic Res Cardiol 97[Suppl 1]:I72–I178
Franzini-Armstrong C, Protasi F (1997) Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev 77:699–729
George CH (2008) Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res 77:302–314. doi:10.1093/cvr/cvm006
Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR (1995) Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest 95:888–894. doi:10.1172/JCI117739
Godt RE, Nosek TM, Maughan DW (1988) Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle on the composition of the cytosol of relaxed skeletal muscle of the frog. J Physiol (Lond) 254:C591–C604
Guo T, Cornea RL, Huke S et al (2010) Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res 106:1743–1752. doi:10.1161/CIRCRESAHA.110.219816
Guo T, Gillespie D, Fill M (2012) Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ Res 111:28–36. doi:10.1161/CIRCRESAHA.112.265652
Gyorke S, Carnes C (2008) Dysregulated sarcoplasmic reticulum calcium release: potential pharmacological target in cardiac disease. Pharmacol Ther 119:340–354. doi:10.1016/j.pharmthera.2008.06.002
Gyorke S, Gyorke I, Lukyanenko V, Terentyev D, Viatchenko-Karpinski S, Wiesner TF (2002) Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front Biosci 7:d1454–d1463
Gyorke I, Hester N, Jones LR, Gyorke S (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86:2121–2128
Haigney MC, Wei S, Kaab S et al (1998) Loss of cardiac magnesium in experimental heart failure prolongs and destabilizes repolarization in dogs. J Am Coll Cardiol 31:701–706
Hasenfuss G, Pieske B (2002) Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34:951–969
Holmberg SR, Williams AJ (1992) The calcium-release channel from cardiac sarcoplasmic reticulum: function in the failing and acutely ischaemic hear. Basic Res Cardiol 87[Suppl 1]:255–268
Hymel L, Inui M, Fleischer S, Schindler H (1988) Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci USA 85:441–445
Ide T, Tsutsui H, Kinugawa S et al (1999) Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85:357–363
Iseri LT, Alexander LC, Mc CR, Boyle AJ, Myers GB (1952) Water and electrolyte content of cardiac and skeletal muscle in heart failure and myocardial infarction. Am Heart J 43:215–227
Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH (2002) Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res 91:1015–1022
Ju YK, Allen DG (1998) Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J Physiol 508(Pt 1):153–166
Ju YK, Allen DG (2007) Store-operated Ca2+ entry and TRPC expression; possible roles in cardiac pacemaker tissue. Heart Lung Circ 16:349–355
Kermode H, Williams AJ, Sitsapesan R (1998) The interactions of ATP, ADP, and inorganic phosphate with the sheep cardiac ryanodine receptor. Biophys J 74:1296–1304
Kinugawa T, Ogino K, Kitamura H et al (1996) Catecholamines, renin-angiotensin-aldosterone system, and atrial natriuretic peptide at rest and during submaximal exercise in patients with congestive heart failure. Am J Med Sci 312:110–117
Kubalova Z, Terentyev D, Viatchenko-Karpinski S et al (2005) Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A 102:14104–14109
Kushnir A, Betzenhauser MJ, Marks AR (2010) Ryanodine receptor studies using genetically engineered mice. FEBS Lett 584:1956–1965. doi:10.1016/j.febslet.2010.03.005
Laver DR (2007) Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys J 92:3541–3555
Laver DR (2009) Luminal Ca2+ activation of cardiac ryanodine receptors by luminal and cytoplasmic domains. Eur Biophys J 39:19–26
Laver DR (2010) Regulation of the RyR channel gating by different modulators. In: Serysheva I (ed) Structure and function of calcium release channels (Current topics in membranes, vol 66). Elsevier, Amsterdam, pp 69–89
Laver DR, Honen BN (2008) Luminal Mg2+, a key factor controlling RYR2-mediated Ca2+ release: cytoplasmic and luminal regulation modeled in a tetrameric channel. J Gen Physiol 132:429–446
Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF (1995) Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membrane Biol 147:7–22
Laver DR, Baynes TM, Dulhunty AF (1997) Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J Membrane Biol 156:213–229
Laver DR, Kong CHT, Imtiaz MS, Cannell MB (2013) Termination of calcium-induced calcium release by induction decay: an emergent property of stochastic channel gating and molecular scale architecture. J Mol Cell Cardiol 54:98–100
Lee YS, Liu OZ, Hwang HS, Knollmann BC, Sobie EA (2013) Parameter sensitivity analysis of stochastic models provides insights into cardiac calcium sparks. Biophys J 104:1142–1150. doi:10.1016/j.bpj.2012.12.055
Lehnart SE, Wehrens XH, Marks AR (2005) Defective ryanodine receptor interdomain interactions may contribute to intracellular Ca2+ leak: a novel therapeutic target in heart failure. Circulation 111:3342–3346
Li J, Imtiaz MS, Beard NA, Dulhunty AF, Thorne R, van Helden DF, Laver DR (2013) ss-Adrenergic stimulation increases RyR2 activity via intracellular Ca2+ and Mg2+ regulation. PLoS One 8:e58334. doi:10.1371/journal.pone.0058334
Li Y, Kranias EG, Mignery GA, Bers DM (2002) Protein kinase a phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res 90:309–316
Lou Q, Fedorov VV, Glukhov AV, Moazami N, Fast VG, Efimov IR (2011) Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure. Circulation 123:1881–1890. doi:10.1161/CIRCULATIONAHA.110.989707
Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM (2003) Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res 92:904–911. doi:10.1161/01.RES.0000069685.20258.F1
Marks AR (2001) Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. J Mol Cell Cardiol 33:615–624
Marks AR (2003) A guide for the perplexed: towards an understanding of the molecular basis of heart failure. Circulation 107:1456–1459
Marks AR, Reiken S, Marx SO (2002) Progression of heart failure: is protein kinase a hyperphosphorylation of the ryanodine receptor a contributing factor? Circulation 105:272–275
Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101:365–376
McGrath KF, Yuki A, Manaka Y, Tamaki H, Saito K, Takekura H (2009) Morphological characteristics of cardiac calcium release units in animals with metabolic and circulatory disorders. J Muscle Res Cell Motil 30:225–231. doi:10.1007/s10974-009-9191-z
Meissner G (1986) Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem 261:6300–6306
Meissner G (1994) Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol 56:485–508. doi:10.1146/annurev.ph.56.030194.002413
Meissner G, Henderson JS (1987) Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem 262:3065–3073
Meissner G, Darling E, Eveleth J (1986) Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry 25:236–244
Mohler PJ, Wehrens XH (2007) Mechanisms of human arrhythmia syndromes: abnormal cardiac macromolecular interactions. Physiology (Bethesda) 22:342–350. doi:10.1152/physiol.00018.2007
Mozaffarian D, Anker SD, Anand I et al (2007) Prediction of mode of death in heart failure: the Seattle heart failure model. Circulation 116:392–398. doi:10.1161/CIRCULATIONAHA.106.687103
Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM, Lamb GD (2013) Ca2+-dependent proteolysis of junctophilin-1 and junctophilin-2 in skeletal and cardiac muscle. J Physiol 591:719–729. doi:10.1113/jphysiol.2012.243279
Nishi M, Sakagami H, Komazaki S, Kondo H, Takeshima H (2003) Coexpression of junctophilin type 3 and type 4 in brain. Brain Res Mol Brain Res 118:102–110
Pathak A, del Monte F, Zhao W et al (2005) Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res 96:756–766. doi:10.1161/01.RES.0000161256.85833.fa
Pedrozo Z, Sanchez G, Torrealba N et al (2010) Calpains and proteasomes mediate degradation of ryanodine receptors in a model of cardiac ischemic reperfusion. Biochim Biophys Acta 1802:356–362. doi:10.1016/j.bbadis.2009.12.005
Piacentino V 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR (2003) Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res 92:651–658. doi:10.1161/01.RES.0000062469.83985.9B
Prestle J, Dieterich S, Preuss M, Bieligk U, Hasenfuss G (1999) Heterogeneous transmural gene expression of calcium-handling proteins and natriuretic peptides in the failing human heart. Cardiovasc Res 43:323–331
Radermacher M, Rao V, Grassucci R, Frank J, Timerman AP, Fleischer S, Wagenknecht T (1994) Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J Cell Biol 127:411–423
Ralston MA, Murnane MR, Kelley RE, Altschuld RA, Unverferth DV, Leier CV (1989) Magnesium content of serum, circulating mononuclear cells, skeletal muscle, and myocardium in congestive heart failure. Circulation 80:573–580
Rardon DP, Cefali DC, Mitchell RD, Seiler SM, Hathaway DR, Jones LR (1990) Digestion of cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles with calpain II. Effects on the Ca2+ release channel. Circ Res 67:84–96
Rigg L, Terrar DA (1996) Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol 81:877–880
Schmidt U, Hajjar RJ, Kim CS, Lebeche D, Doye AA, Gwathmey JK (1999) Human heart failure: cAMP stimulation of SR Ca2+-ATPase activity and phosphorylation level of phospholamban. Am J Physiol 277:H474–H480
Schwinger RH, Bohm M, Schmidt U et al (1995) Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation 92:3220–3228
Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E (1999) Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol 31:479–491
Shan J, Betzenhauser MJ, Kushnir A et al (2010) Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest 120:4375–4387. doi:10.1172/JCI37649
Shannon TR, Pogwizd SM, Bers DM (2003) Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93:592–594
Sitsapesan R, Williams AJ (1994a) Gating of the native and purified cardiac SR Ca2+-release channels with monovalent cations as permeant species. Biophys J 67:1484–1494
Sitsapesan R, Williams AJ (1994b) Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by luminal Ca2+. J Membr Biol 137:215–226
Sitsapesan R, Williams AJ (1997) Regulation of current flow through ryanodine receptors by luminal Ca2+. J Membr Biol 159:179–185
Smith JS, Coronado R, Meissner G (1986) Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol 88:573–588
Sobie EA, Song LS, Lederer WJ (2005) Local recovery of Ca2+ release in rat ventricular myocytes. J Physiol 565:441–447. doi:10.1113/jphysiol.2005.086496
Soeller C, Cannell MB (1997) Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys J 73:97–111. doi:10.1016/S0006-3495(97)78051-2
Song LS, Pi Y, Kim SJ et al (2005) Paradoxical cellular Ca2+ signaling in severe but compensated canine left ventricular hypertrophy. Circ Res 97:457–464. doi:10.1161/01.RES.0000179722.79295.d4
Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H (2006) Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA 103:4305–4310. doi:10.1073/pnas.0509324103
Studer R, Reinecke H, Bilger J et al (1994) Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure. Circ Res 75:443–453
Sun J, Xin C, Eu JP, Stamler JS, Meissner G (2001) Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci USA 98:11158–11162
Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K (2000) Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell 6:11–22
Tijskens P, Jones LR, Franzini-Armstrong C (2003) Junctin and calsequestrin overexpression in cardiac muscle: the role of junctin and the synthetic and delivery pathways for the two proteins. J Mol Cell Cardiol 35:961–974
Tripathy A, Meissner G (1996) Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J 70:2600–2615
van Helden DF (1993) Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol 471:465–479
van Helden DF, Imtiaz MS (2003) Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol (Lond) 548:271–296
Vinogradova TM, Maltsev VA, Bogdanov KY, Lyashkov AE, Lakatta EG (2005) Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann N Y Acad Sci 1047:138–156
Walweel K, Li J, Molenaar P et al (2014) Differences in the regulation of RyR2 from human, sheep, and rat by Ca2+ and Mg2+ in the cytoplasm and in the lumen of the sarcoplasmic reticulum. J Gen Physiol 144:263–271. doi:10.1085/jgp.201311157
Wehrens XH, Lehnart SE, Huang F et al (2003) FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113:829–840
Wehrens XH, Lehnart SE, Reiken SR et al (2004) Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304:292–296
Wehrens XH, Lehnart SE, Marks AR (2005) Ryanodine receptor-targeted anti-arrhythmic therapy. Ann N Y Acad Sci 1047:366–375
Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR (2006) Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA 103:511–518
Xu L, Mann G, Meissner G (1996) Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res 79:1100–1109
Xu L, Eu JP, Meissner G, Stamler JS (1998) Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279:234–237
Yano M, Okuda S, Oda T et al (2005) Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation 112:3633–3643
Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 272:23389–23397
Zima AV, Picht E, Bers DM, Blatter LA (2008) Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res 103:e105–e115. doi:10.1161/CIRCRESAHA.107.183236
Zima AV, Bovo E, Bers DM, Blatter LA (2010) Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol 588:4743–4757. doi:10.1113/jphysiol.2010.197913
Zissimopoulos S, Docrat N, Lai FA (2007) Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem 282:6976–6983
Acknowledgements
This work was supported by a scholarship from the University of Newcastle and the Hunter Medical Research Institute and a project grant from the NH&MRC (631052).
Compliance with Ethical Standards
ᅟ
Conflict of interest
Kafa Walweel declares that she has no conflict of interest Derek R. Laver declares that he has no conflict of intrest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: Biophysics of Human Heart Failure
Rights and permissions
About this article
Cite this article
Walweel, K., Laver, D.R. Mechanisms of SR calcium release in healthy and failing human hearts. Biophys Rev 7, 33–41 (2015). https://doi.org/10.1007/s12551-014-0152-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-014-0152-4