Abstract
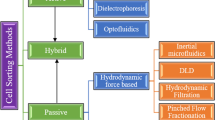
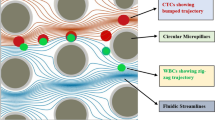
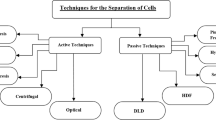
Deterministic lateral displacement (DLD) is evolving as an effective passive technique for seclusion of circulating tumor cells (CTCs) functioning based on nonuniform splitting of laminar flow moving through an array of micropillars. In this research work, an unconventional approach has been presented to alter the fluidic resistance between micropillars in asymmetric DLD array for better separation of CTCs in a blood sample. This paper is aimed at introducing an innovative approach using electrical network analogy for tuning of fluidic resistance resulting in enhanced seclusion of CTCs from WBCs implementing the concept of asymmetric DLD array. The paper also describes the computational analysis of a microfluidic device using tuned asymmetric DLD array technique. A cognitive clinical decision support system for identification of CTCs based on the model is also illustrated. In this paper, computational fluid dynamics approach has been used through simulation of the microfluidic device in COMSOL Multiphysics 5.4 software to effectively regulate the trajectory of differently sized CTCs and WBCs. A novel mathematical fluidic resistance tuning approach has been introduced to design the DLD array for effective segregation of different varieties of CTCs realized by computational visualization of trajectory working on Navier–Stokes equation. The proposed design of microfluidic device isolates three distinct CTCs, i.e., lung cancer CTCs, prostate cancer CTCs, and breast cancer CTCs of diameters 22.5 µm, 10.64 µm, and 13.1 µm, respectively, from tiny WBCs of diameter 12 µm with separation efficiencies above 90% at a high sample flow rate of 20 × 10−6 kg/s, thereby offering higher throughput. The tuning model of fluidic resistances between micropillars has been shown to offer minimal resistive effect to the required CTC trajectory while maintaining uniform pressure distribution around micropillars.















Similar content being viewed by others
References
Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP, Smirnov DA. Microfluidics and circulating tumor cells. J Mol Diagn. 2013;15(2):149–57.
Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol 2010;617421.
Bouche O, Beretta GD, Alfonso PG, Geissler M. The role of anti-epidermal growth factor receptor monoclonal antibody monotherapy in the treatment of metastatic colorectal cancer. Cancer Treat Rev. 2010;36(Suppl 1):S1–10.
den Toonder J. Circulating tumor cells: the Grand Challenge. Lab Chip. 2011;11:375–7.
Mahmud M, Kaiser MS, McGinnity TM, Hussain A. Deep learning in mining biological data. Cogn Comput. 2021;13(1):1–33.
Faundez-Zanuy M, Fierrez J, Ferrer MA, Diaz M, Tolosana R, Plamondon R. Handwriting biometrics: Applications and future trends in e-security and e-health. Cogn Comput. 2020;12(5):940–53.
Deebak BD, Al-Turjman F. Smart mutual authentication protocol for cloud based medical healthcare systems using Internet of medical things. IEEE Journal on Selected Areas in Communications. 2020.
Jin, et al. Technologies for label-free separation of circulating tumor cells: from historical foundations to recent developments. Lab Chip. 2014;14(1):32–44.
Meng, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10(24):8152–62.
Park S, et al. Morphological differences between circulating tumor cells from prostate cancer patients and cultured prostate cancer cells. PLoS One. 2014;9(1):e85264.
Allard WJ, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–904.
Thiele J, Bethel K, Králíčková M, Kuhn P. Circulating tumor cells: fluid surrogates of solid tumors. Annu Rev Pathol. 2017;12:419–47.
Yang M, et al. Incorporating blood-based liquid biopsy information into cancer staging: time for a TNMB system? Ann Oncol. 2017;29(2):311–23.
Songjaroen T, Dungchai W, Chailapakul O, Henry CS, Laiwattanapaisal W. Blood separation on microfluidic paper-based analytical devices. Lab Chip. 2012;12:3392–8.
Kim J-H, Woenker T, Adamec J, Regnier FE. Simple, miniaturized blood plasma extraction method. Anal Chem. 2013;85:11501–8.
Haeberle S, Brenner T, Zengerle R, Ducrée J. Centrifugal extraction of plasma from whole blood on a rotating disk. Lab Chip. 2006;6:776–81.
Amasia M, Madou M. Large-volume centrifugal microfluidic device for blood plasma separation. Bioanalysis. 2010;2:1701–10.
Kersaudy-Kerhoas M, Sollier E. Micro-scale blood plasma separation: from acoustophoresis to egg-beaters. Lab Chip. 2013;13:3323–46.
Witek MA, Freed IM, Soper SA. Cell separations and sorting. Anal Chem. 2019;92(1):105–31.
Ishikawa T, Fujiwara H, Matsuki N, Yoshimoto T, Imai Y, Ueno H, Yamaguchi T. Asymmetry of blood flow and cancer cell adhesion in a microchannel with symmetric bifurcation and confluence. Biomed Microdevices. 2011;13:159–67.
Karimi A, Yazdi S, Ardekani AM. Hydrodynamic mechanisms of cell and particle trapping in microfluidics. Biomicrofluidics. 2013;7:21501.
Zhang J, Yan S, Yuan D, Alici G, Nguyen NT, Warkiani ME, Li W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip. 2016;16:10–34.
Pødenphant M1, Ashley N, Koprowska K, Mir KU, Zalkovskij M, Bilenberg B, Bodmer W, Kristensen A, Marie R. Separation of cancer cells from white blood cells by pinched flow fractionation. Lab Chip. 2015;15(24):4598–4606.
Yoon Y, Lee J, Ra M, Gwon H, Lee S, Kim MY, Yoo K-C, Sul O, Kim CG, Kim W-Y, Park J-G, Lee S-J, Lee YY, Choi HS, Lee S-B. Continuous separation of circulating tumor cells from whole blood using a slanted weir microfluidic device. Cancers. 2019;11(2):200.
Dharmasiri U, Njoroge SK, Witek MA, Adebiyi MG, Kamande JW, Hupert ML, Barany F, Soper SA. High-throughput selection, enumeration, electrokinetic manipulation, and molecular profiling of low-abundance circulating tumor cells using a microfluidic system. Anal Chem. 2011;83(6):2301–9.
Johnson ES, Anand RK, Chiu DT. Improved detection by ensemble-decision aliquot ranking of circulating tumor cells with low numbers of a targeted surface antigen. Anal Chem. 2015;87(18):9389–95.
Kumar V, Rezai P. Magneto-hydrodynamic fractionation (MHF) for continuous and sheathless sorting of high-concentration paramagnetic microparticles. Biomed Microdevices. 2017;19:39.
Salafi T, Zhang Y, Zhang Y. A review on deterministic lateral displacement for particle separation and detection. Nano-Micro Lett. 2019;11:77.
Kottmeier J. Maike Wullen weber, Sebastian Blahout, Jeanette Hussong, Ingo Kampen, Arno Kwade and Andreas Dietzel, Accelerated particle separation in a DLD device at Re > 1 investigated by means of µPIV. Micromachines. 2019;10:768.
Zeming K, Salafi T, Chen C, et al. Asymmetrical deterministic lateral displacement gaps for dual functions of enhanced separation and throughput of red blood cells. Sci Rep. 2016;6:22934.
Inglis D, Vernekar R, Krüger T, et al. The fluidic resistance of an array of obstacles and a method for improving boundaries in deterministic lateral displacement arrays. Microfluid Nanofluid. 2020;24(3):18.
Fachin F, Spuhler P, Martel-Foley JM, et al. Monolithic chip for high-throughput blood cell depletion to sort rare circulating tumor cells. Sci Rep. 2017;7:10936.
Phillips KG, et al. Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Front Oncol. 2012;2:72.
Zhou J, Kulasinghe A, Bogseth A, et al. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst Nanoeng. 2019;5(8).
Qin L, et al. Highly Efficient Isolation of Circulating Tumor Cells Using a Simple Wedge-Shaped Microfluidic Device. IEEE Trans Biomed Eng. 2019;66(6):1536–41.
Liu Z, Chen R, Li Y, Liu J, Wang P, Xia X, Qin L. Integrated microfluidic chip for efficient isolation and deformability analysis of circulating tumor cells. Adv Biosyst. 2018;2(10):1800200.
González-Esparza D, Del Angel-Arroyo JA, Elvira-Hernández EA, Herrera-May AL, Aguilera-Cortés LA. Design and modeling of a microfluidic device with potential application for isolation of circulating tumor cells. IEEE International Conference on Engineering Veracruz (ICEV), Boca del Rio, Veracruz, Mexico. 2019;1–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhattacharjee, R., Kumar, R. & Al-Turjman, F. A Novel Approach for Tuning of Fluidic Resistance in Deterministic Lateral Displacement Array for Enhanced Separation of Circulating Tumor Cells. Cogn Comput 14, 1660–1676 (2022). https://doi.org/10.1007/s12559-021-09904-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12559-021-09904-y