Abstract
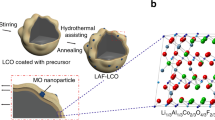
Iron-substituted cobalt-free lithium-rich manganese-based materials, with advantages of high specific capacity, high safety, and low cost, have been considered as the potential cathodes for lithium ion batteries. However, challenges, such as poor cycle stability and fast voltage fade during cycling under high potential, hinder these materials from commercialization. Here, we developed a method to directly coat LiF on the particle surface of Li1.2Ni0.15Fe0.1Mn0.55O2. A uniform and flat film was successfully formed with a thickness about 3 nm, which can effectively protect the cathode material from irreversible phase transition during the deintercalation of Li+. After surface coating with 0.5wt% LiF, the cycling stability of Li1.2Ni0.15Fe0.1Mn0.55O2 cycled at high potential was significantly improved and the voltage fade was largely suppressed.
Similar content being viewed by others
References
R. Schmuch, R. Wagner, G. Hörpel, T. Placke, and M. Winter, Performance and cost of materials for lithium-based rechargeable automotive batteries, Nat. Energy, 3(2018), No. 4, p. 267.
A. Kwade, W. Haselrieder, R. Leithoff, A. Modlinger, F. Dietrich, and K. Droeder, Current status and challenges for automotive battery production technologies, Nat. Energy, 3(2018), No. 4, p. 290.
P.K. Nayak, E.M. Erickson, F. Schipper, et al., Review on challenges and recent advances in the electrochemical performance of high capacity Li- and Mn-rich cathode materials for Li-ion batteries, Adv. Energy Mater., 8(2018), No. 8, art. No. 1702397.
M.S. Whittingham, Lithium batteries and cathode materials, Chem. Rev., 104(2004), No. 10, p. 4271.
J.M. Zheng, S. Myeong, W. Cho, et al., Li- and Mn-rich cathode materials: Challenges to commercialization, Adv. Energy Mater., 7(2017), No. 6, art. No. 1601284.
S. Hy, F. Felix, J. Rick, W.N. Su, and B.J. Hwang, Direct in situ observation of Li2O evolution on Li-rich high-capacity cathode material, Li[NixLi(1−2x)/3Mn(2−x)/3]O2 (0 ≤ x ≤ 0.5), J. Am. Chem. Soc., 136(2014), No. 3, p. 999.
M.M. Thackeray, S.H. Kang, C.S. Johnson, J.T. Vaughey, R. Benedek, and S.A. Hackney, Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries, J. Mater. Chem., 17(2007), No. 30, p. 3112.
A.D. Robertson and P.G. Bruce, Mechanism of electrochemical activity in Li2MnO3, Chem. Mater., 15(2003), No. 10, p. 1984.
R. Robert, C. Villevieille, and P. Novák, Enhancement of the high potential specific charge in layered electrode materials for lithium-ion batteries, J. Mater. Chem. A, 2(2014), No. 23, p. 8589.
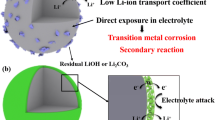
C. Zhan, T.P. Wu, J. Lu, and K. Amine, Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes - A critical review, Energy Environ. Sci., 11(2018), No. 2, p. 243.
J.M. Zheng, M. Gu, J. Xiao, P.J. Zuo, C.M. Wang, and J.G. Zhang, Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process, Nano Lett., 13(2013), No. 8, p. 3824.
M. Xu, Z.Y. Chen, H.L. Zhu, X.Y. Yan, L.J. Li, and Q.F. Zhao, Mitigating capacity fade by constructing highly ordered mesoporous Al2O3/polyacene double-shelled architecture in Li-rich cathode materials, J. Mater. Chem. A, 3(2015), No. 26, p. 13933.
B.W. Xiao and X.L. Sun, Surface and subsurface reactions of lithium transition metal oxide cathode materials: An overview of the fundamental origins and remedying approaches, Adv. Energy Mater., 8(2018), No. 29, art. No. 1802057.
Y.P. Gan, Y.S. Wang, J.F. Han, et al., Synthesis and electrochemical performance of nano TiO2(B)-coated Li[Li0.2Mn0.54Co0.13Ni0.13]O2 cathode materials for lithium-ion batteries, New J. Chem., 41(2017), No. 21, p. 12962.
J.M. Zheng, M. Gu, J. Xiao, et al., Functioning mechanism of AlF3 coating on the Li- and Mn-rich cathode materials, Chem. Mater., 26(2014), No. 22, p. 6320.
F. Wu, J.R. Liu, L. Li, et al., Surface modification of Li-rich cathode materials for lithium-ion batteries with a PEDOT: PSS conducting polymer, ACS Appl. Mater. Interfaces, 8(2016), No. 35, p. 23095.
Z.K. Zhao, H.L. Xie, Z.Y. Wen, et al., Tuning Li3PO4 modification on the electrochemical performance of nickel-rich LiNi0.6Co0.2Mn0.2O2, Int. J. Miner. Metall. Mater., 28(2021), No. 9, p. 1488.
Y. Yamada J.H. Wang, S. Ko, E. Watanabe, and A. Yamada, Advances and issues in developing salt-concentrated battery electrolytes, Nat. Energy, 4(2019), No. 4, p. 269.
X. Cao, X.D. Ren, L.F. Zou, et al., Monolithic solid-electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization, Nat. Energy, 4(2019), No. 9, p. 796.
C.V. Amanchukwu, X. Kong, J. Qin, Y. Cui, and Z.N. Bao, Nonpolar alkanes modify lithium-ion solvation for improved lithium deposition and stripping, Adv. Energy Mater., 9(2019), No. 41, art. No. 1902116.
Z.A. Yu, H.S. Wang, X. Kong, et al., Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries, Nat. Energy, 5(2020), No. 7, p. 526.
B.P. Thapaliya, S. Misra, S.Z. Yang, et al., Enhancing cycling stability and capacity retention of NMC811 cathodes by reengineering interfaces via electrochemical fluorination, Adv. Mater. Interfaces, (2022), art. No. 2200035.
L.F. Wang, M.M. Geng, X.N. Ding, et al., Research progress of the electrochemical impedance technique applied to the high-capacity lithium-ion battery, Int. J. Miner. Metall. Mater., 28(2021), No. 4, p. 538.
W. Liu, J.X. Li, W.T. Li, H.Y. Xu, C. Zhang, and X.P. Qiu, Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte, Nat. Commun., 11(2020), art. No. 3629.
J.W. Min, J. Gim, J.J. Song, et al., Simple, robust metal fluoride coating on layered Li1.23Ni0.13Co0.14Mn0.56O2 and its effects on enhanced electrochemical properties, Electrochim. Acta, 100(2013), p. 10.
X.L. Cheng, H.Z. Wei, W.J. Hao, et al., A cobalt-free Li(Li0.16Ni0.19Fe0.18Mn0.46)O2 cathode for lithium-ion batteries with anionic redox reactions, ChemSusChem, 12(2019), No. 6, p. 1162.
Acknowledgement
This work was financially supported by the project of International Science & Technology Cooperation of China (No. 2019YFE0100200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Liu, W., Li, J., Xu, H. et al. Stabilized cobalt-free lithium-rich cathode materials with an artificial lithium fluoride coating. Int J Miner Metall Mater 29, 917–924 (2022). https://doi.org/10.1007/s12613-022-2483-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-022-2483-7