Abstract
Purpose
AuraGainTM, a novel third-generation laryngeal mask, can facilitate insertion of a gastric tube and provide the potential advantage of intubation. Data are lacking on intubation through the AuraGain laryngeal mask.
Methods
Eighty-eight hip or knee surgery patients were enrolled in this parallel randomized-controlled trial. We hypothesized that intubation time using the AuraGain laryngeal mask would be no longer than that for standard flexible bronchoscopic intubation over a slit Guedel tube, with a non-inferiority margin of five seconds. The following data were recorded during a maximum of three intubation attempts: intubation time, number of intubation attempts, degree of resistance to advance the endotracheal tube, and mask placement (i.e., Brimacombe score). Follow-up outcomes, including neck pain, hoarseness, and dysphagia, were also measured two and 24 hr postoperatively. Patients and outcome assessors remained blinded until the last examination.
Results
Mean intubation time was similar between the Guedel tube and AuraGain groups (23.6 sec vs 21.4 sec, respectively). The upper limit of the 95% confidence interval (CI) of the difference in mean intubation time between groups fell below our pre-specified non-inferiority margin; therefore, we found the AuraGain laryngeal mask to be non-inferior to the slit Guedel tube (adjusted group difference, −1.6 sec; 95% CI, −3.7 to 0.5). Successful intubation was achieved in the majority of patients (≥ 95%) in each group on the first attempt. No resistance to insertion of the endotracheal tube was encountered in the majority of patients in each group, and no complications were reported during the 24-hr postoperative period. There was no difference in the Brimacombe score or in the status of postoperative morbidity between the two groups.
Conclusion
We conclude that flexible bronchoscopic intubation through an AuraGain laryngeal mask can be achieved at least as fast as standard bronchoscopic intubation without contributing to additional patient morbidity or postoperative discomfort.
Trial registration
www.clinicaltrials.gov, NCT 02570269. Registered 23 September 2015.
Résumé
Objectif
Le dispositif AuraGainTM est un nouveau masque laryngé de troisième génération qui peut faciliter l’insertion d’une sonde gastrique et offrir l’avantage potentiel de l’intubation. Les données concernant l’intubation via un masque laryngé AuraGain sont rares.
Méthode
Quatre-vingt-huit patients devant subir une chirurgie de la hanche ou du genou ont participé à cette étude randomisée contrôlée parallèle. Nous avons émis l’hypothèse que le temps nécessaire à l’intubation avec un masque laryngé AuraGain ne serait pas plus long que celui nécessaire à une intubation standard réalisée à l’aide d’un bronchoscope flexible sur une canule de Guedel fendue, avec une marge de non-infériorité de cinq secondes. Les données suivantes ont été enregistrées au cours d’un maximum de trois tentatives d’intubation : temps d’intubation, nombre de tentatives d’intubation, degré de résistance à l’insertion de la sonde endotrachéale et positionnement du masque (c.-à-d. score de Brimacombe). Les critères de suivi, notamment la douleur au niveau du cou, l’enrouement et la dysphagie, ont également été mesurés à deux et 24 h après l’opération. L’assignation des groupes a été maintenue en aveugle pour les patients et les évaluateurs jusqu’au dernier examen clinique.
Résultats
Le temps d’intubation moyen était semblable dans le groupe canule de Guedel et le groupe AuraGain (23,6 sec vs 21,4 sec, respectivement). La limite supérieure de l’intervalle de confiance (IC) à 95 % de la différence en temps d’intubation moyen entre les groupes se situait au-dessous de notre marge de non-infériorité préalablement spécifiée; nous avons donc constaté que le masque laryngé AuraGain était non inférieur à la canule de Guedel fendue (différence ajustée entre les groupes, -1,6 sec; IC 95 %, -3,7 à 0,5). L’intubation a réussi à la première tentative chez la majorité des patients (≥ 95 %) de chaque groupe. Chez la majorité des patients des deux groupes, aucune résistance à l’insertion de la sonde endotrachéale n’a été observée, et aucune complication n’a été rapportée au cours de la période postopératoire de 24 h. Aucune différence n’est apparue dans le score de Brimacombe ou le statut de morbidité postopératoire entre les deux groupes.
Conclusion
Nous concluons qu’une intubation réalisée avec un bronchoscope flexible via un masque laryngé AuraGain peut être réalisée au moins aussi rapidement qu’une intubation standard avec bronchoscope, sans pour autant ajouter à la morbidité ou à l’inconfort postopératoire des patients.
Enregistrement de l’étude
www.clinicaltrials.gov, NCT 02570269. Enregistrée le 23 septembre 2015.
Similar content being viewed by others
Difficult airway management remains one of the important topics in anesthesia, with a 4.5-7.5% incidence in difficult intubation.1 According to airway management guidelines, a supraglottic airway is the first rescue device for the unanticipated difficult airway.2 If ventilation cannot be sufficiently performed through a supraglottic airway, the next step most often requires tracheal intubation.
Various supraglottic airways, e.g., the second-generation single-use i-gel™ (Intersurgical Ltd, Wokingham, UK) or the newer Ambu® AuraGain™ (Ambu A/S, Ballerup, Denmark), allow insertion of an endotracheal tube (ETT) through the device3,4 (Fig. 1). Timely placement of an ETT can be critical in the situation of a difficult airway and plays an important role in difficult airway algorithms.5,6 Flexible bronchoscopic intubation through a slit Guedel tube remains one of the most common practices in expected difficult intubation, yet bronchoscopic intubation via a supraglottic airway may now be considered an alternative approach.2,7,8,9,10 This is an easier alternative for the management of the difficult airway, as it offers younger less experienced anesthesia providers an easier handling method to gain sufficient and safe practice in bronchoscopic awake intubation.11 Orotracheal bronchoscopic intubation may be facilitated by diverse airway or supraglottic devices, such as the slit Guedel tube or the Ovassapian airway (Hudson RCI®), which help guide the flexible bronchoscope to the glottic opening.12 Scientific evidence is lacking regarding the ease of bronchoscopic placement of an ETT through the AuraGain and the associated morbidity and discomfort for the patient following this intubation procedure.
We hypothesized that no additional time would be needed for AuraGain intubation compared with the time for the standard intubation procedure using a Guedel tube. To test this hypothesis, we compared the bronchoscopic intubation procedure of the slit Guedel tube with that of the newer generation AuraGain laryngeal mask in a non-inferiority parallel single-blind randomized-controlled trial.
Methods
This randomized-controlled trial was initiated after approval was granted by the Cantonal Ethics Committee of Zurich, Switzerland (KEK-ZH Nr. 2015-0510). This manuscript adheres to the Consolidated Standards of Reporting Trials (CONSORT) statement.13
Patient selection and randomization
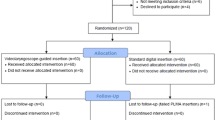
This non-inferiority single-blind randomized-controlled trial was conducted at the Schulthess Klinik from December 2015-January 2016. We screened 88 consecutive patients undergoing elective knee or hip surgery for study inclusion. Patients aged ≥ 18 yr with the following criteria were excluded: American Society of Anesthesiologists (ASA) physical status ≥ III; body mass index > 35 kg⋅m−2; indication for a rapid sequence induction, such as known reflux or full stomach; previous operations or diseases of the airway or neck; contraindications to the use of a supraglottic airway; diseases affecting the suitability of the anesthesia or preventing accurate postoperative clinical examination; alcohol intoxication; previous drug abuse; or legal incompetence. After providing written informed consent, patients were randomly assigned to receive intubation using the conventional top-slit oropharyngeal airway, the slit Guedel tube (Rüsch & Co, Kernen, Germany), or the AuraGain laryngeal mask.
A block randomization sequence was generated using the Stata® (StataCorp LP, College Station, TX, USA) command “ralloc”14 and administered electronically within the project-protected on-line database using REDCap.15 Shortly before each operation, the anesthesiologist logged into the database to obtain the next allocated intubation group in sequence. For obvious reasons, anesthesiologists were not blinded to the allocated groups. Patients and outcome assessors, however, remained blinded to their allocated group until completion of the last follow-up examination.
Prior to surgery, patients reported their smoking and drinking habits as well as whether they suffered from any neck pain (according to a 0-10 numeric rating scale [NRS]) and/or the presence of hoarseness, sore throat, or other neck pathologies.
Intubation procedure and airway management
Each intubation was performed by one of three experienced anesthesiologists (B.M., C.K., H.R.B.), each with expertise in at least 20 and 500 oropharyngeal bronchoscopic intubations using the AuraGain and Guedel tubes, respectively. Upon the patient’s arrival in the anesthesia room, standard monitoring was established.16 All patients received preoxygenation for three minutes before anesthesia was induced with fentanyl 2-4 µg⋅kg−1 and propofol 2.5-3.0 mg⋅kg−1 administered within 20-30 sec. Rocuronium 0.5 mg⋅kg−1 was given as the neuromuscular blockade. Curarization was monitored with a TOF-Watch® (Organon Ltd, Dublin, Ireland), and the patient’s head was then placed in a neutral position. The Guedel tube or AuraGain device was inserted once adequate lung ventilation was confirmed by appropriate chest excursions and capnography curves showing no excessive leakage. Anesthesia was maintained during surgery with remifentanil 0.1-0.3 µg⋅kg−1·min−1 and propofol 4-6 mg⋅kg−1·hr−1while breathing 60% oxygen.
For all patients, we used a Unoflex™ reinforced ETT with an internal diameter of 7 mm. Airway management differed between the two intubation groups. For the Guedel tube group, the size (length) of the device was set according to the measured distance from earlobe to the angle of the mouth.5 The Guedel tube was inserted as described by Kim et al., i.e., with its tip facing upwards while passing through the patient’s mouth, followed by a 180° rotation downwards while advancing into the throat.17 With the Guedel tube in place, the patient’s lungs were ventilated with a face mask, 100% oxygen, and a flow of 6 L⋅min−1. Pressure-controlled ventilation was maintained over the face mask with a frequency of 14 breaths⋅min−1 and a pressure of 15 mbar (12 mmHg). A flexible bronchoscope with a diameter of 4.8 mm (Olympus BF-Q190; Volketswil; Switzerland) was mounted with a Unoflex reinforced ETT (internal diameter, 7 mm) (ConvaTec Ltd, Flintshire, UK). Prior to applying the bronchoscope, the Guedel tube was lubricated with K-Y® jelly (Johnson & Johnson, Sezanne, France) to improve bronchoscope insertion. Ventilation was suspended, and the oropharyngeal space was kept open using a jaw-thrust maneuver. The tip of the bronchoscope was advanced through and towards the lower end of the Guedel tube. Continuing past the vocal cords with a view of the carina, the ETT was advanced over the bronchoscope into the trachea after removing the Guedel tube. Positioning the ETT was controlled with the bronchoscope at the lower end of the ETT, placing the tube 2-3 cm above the carina. After bronchoscope removal, the ETT cuff was inflated with 30 cm H2O with a manual manometer.
For the AuraGain group, the size of the device was selected based on the patient’s weight according to the manufacturer`s recommendations. The airway channel of the mask was lubricated with K-Y jelly and the cuff was fully deflated. Ventilation was stopped, the oropharyngeal space was kept open with the jaw thrust maneuver, and the AuraGain was inserted following manufacturer`s instructions. The cuff was inflated with a manometer to 60 cm H2O once the AuraGain device was correctly positioned, and the patient’s lungs were then ventilated manually until capnography curve analysis confirmed normal ventilation. The device was then fixed to the patient’s face. Ventilation and respiration were regulated with the Fabius Tiro® system (Dräger Schweiz AG, Liebefeld, Switzerland) using a breathing frequency of 14 breaths⋅min−1 and a starting pressure of 15 mbar (12 mmHg). At this time, an esophageal tube was inserted into the drain tube and gastric content was suctioned. The ease of advancing the esophageal tube was recorded. In a similar manner to the Guedel tube group, the 4.8-mm Olympus bronchoscope mounted with the ETT was inserted through the AuraGain into the trachea. The ETT was advanced into the trachea with the bronchoscope at its lower end until its position was 2-3 cm above the carina or until the AuraGain stopped the proximal end of the tracheal tube (Fig. 1). After removal of the bronchoscope, the ETT cuff was inflated with 30 cm H2O, and ventilation was started with the AuraGain in situ.
In both groups, adequate insertion of the ETT was confirmed by detection and by capnography curve analysis of the exhaled gas and bilateral lung auscultation. Tracheal extubation was performed in all patients according to standard clinical practice on completion of the surgical procedure.
Patient follow-up
The primary outcome was defined as the overall time to intubate with the AuraGain compared with that using standard bronchoscopic intubation over a slit Guedel tube. Secondary outcomes were the number of attempts for successful intubation, ease of intubation, bronchoscopic positioning using a flexible bronchoscope to visualize the glottic opening, as well as hoarseness, sore throat, or dysphagia.
In the Guedel tube group, intubation time measured in seconds began once the bronchoscope tip advanced through the Guedel tube and ended when the bronchoscope tip left the ETT. For AuraGain, intubation time was the interval from when the bronchoscope tip entered the proximal end of the laryngeal mask to when it finally exited the AuraGain. The time to complete the procedure was noted if intubation was successful on the first attempt. Resistance encountered while railroading the ETT over the bronchoscope through the supraglottic device was scored with the four-point system adopted from Blair et al. 18 When intubation failed, two further attempts were permitted while ventilation continued over the face mask (Guedel tube group) or AuraGain (AuraGain group).
With the bronchoscope at the lower end of the Guedel tube or as soon as the ETT was inserted through the AuraGain into the trachea, we determined the position of the bronchoscopic view of the vocal cords according to the established Brimacombe score (4 = only vocal cords visible; 3 = vocal cords plus posterior epiglottis visible; 2 = vocal cords plus anterior epiglottis visible; 1= vocal cords not seen).19 In the AuraGain group, if the vocal cords were not visible (Brimacombe score 1) or if ventilation was not possible over the AuraGain, the anesthesiologist did not attempt to advance the tracheal tube. The duration of surgery was measured.
Two hours after surgery and again at the 24-hr follow-up time point, a blinded anesthesiologist asked all patients whether they experienced any neck pain according to the 0-10 NRS, hoarseness, sore throat, or dysphagia. Adverse events that occurred during and up to 24 hr postoperatively were recorded.
Statistical analysis
This study was designed to show the non-inferiority of AuraGain intubation time compared with that of the Guedel tube. We calculated a sample size of 44 patients per group a priori based on a 1:1 treatment allocation, considering a non-inferiority margin of five seconds and a standard deviation of eight seconds (unpublished pilot data using the Guedel tube), a significance level of 0.05 (type I error), and a power of 90% (type II error 0.10).20
Statistical analyses were performed using Stata version 13 (StataCorp LP, College Station, TX, USA). Baseline parameters were tabulated separately per group using standard descriptive statistics and compared using clinical judgement to assess differences that could influence the study outcomes. Outcome parameters were also tabulated per group as well as per examination time point.
The primary outcome of intubation time was analyzed by quantifying the group difference in means and its 95% confidence interval (CI) using regression analyses with and without adjusting for age and sex differences between the groups. The decision for statistical adjustment was made after comparing baseline parameters between the groups. Secondary analyses were performed using Fisher’s exact test to assess differences in categorical outcomes at each follow-up time and were exploratory.
Results
Patient recruitment, treatment allocation, and follow-up
Ninety-four patients provided informed consent and were enrolled and randomized from December 2015-January 2016 (Fig. 1, CONSORT flow diagram). After six post-randomization exclusions involving four Guedel tube patients and two patients from the AuraGain intervention group, there were 43 and 45 patients allocated to the Guedel tube and AuraGain interventions, respectively. There were no patient dropouts up to and including the final 24-hr postoperative examination.
Baseline group comparison and intubation parameters
Both intervention groups were similar regarding the preoperative baseline parameters (Table 1). There was a group difference in the mean patient age of −4.9 yr (95% CI, −10.5 to 7.0), and the percentage of female patients in the Guedel tube group was 65% compared with 47% in the AuraGain group. Overall, the majority of patients were non-smokers, ASA physical status II, and with no pain or hoarseness prior to surgery.
Follow-up outcomes
The mean intubation time was 23.6 sec and 21.4 sec in the Guedel tube and AuraGain groups, respectively, with an adjusted group difference of −1.6 sec (95% CI, −3.7 to 0.5) (Table 2). The unadjusted group difference was −2.2 sec (95% CI, −4.2 to −0.1). Intubation was successful in the majority of patients (96% in the AuraGain group and 95% in the Guedel tube group) on the first attempt. In the Guedel tube group, we had two successful second attempts at intubation compared with one successful second attempt with the AuraGain. Intubation was not successful in one patient on the third attempt, but this patient’s lungs were successfully ventilated with the AuraGain.
A resistance-free passage of the ETT through the vocal cords occurred in 70% of the Guedel tube group vs 80% of the AuraGain group (Table 2). In addition, marked resistance on advancing the ETT was noted in two patients in the Guedel tube group vs one patient in the AuraGain group.
The Brimacombe score differed slightly between the two intervention groups (Table 2). Only four Guedel tube patients had a score of 4 compared with 12 patients in the AuraGain group, and 14 patients had a Brimacombe score of 2 in the Guedel tube group compared with only nine patients in the AuraGain group. Nevertheless, there was a similar proportion of patients with a Brimacombe score of 3 in both groups. The total surgical time was close to 80 min for both groups.
There were no significant differences in neck pain between the Guedel tube and AuraGain groups at two and 24 hr after surgery (Table 3). Notably, four and two AuraGain patients had 24-hr postoperative NRS scores of 1 and 2, respectively, and a further AuraGain patient rated an increase in neck pain (i.e., NRS = 3) at the final postoperative examination. At both postoperative follow-ups, over 90% and 70% of patients from the Guedel tube and AuraGain cohorts, respectively, experienced no difficulty in swallowing and no hoarseness (Table 3). Finally, we did not perform any esophageal intubations and did not experience any complications during all intubation procedures.
Discussion
This randomized-controlled trial showed non-inferiority of the AuraGain compared with the Guedel tube regarding intubation time. Although two Guedel tube patients could not be evaluated due to logistic issues with the bronchoscope at the time of surgery, the majority of enrolled patients were similar in their baseline characteristics, with minor differences in age and sex distributions that were accounted for in the statistical analyses.
The confidence interval of the difference in intubation time between the two interventions ranged from -3.7 to 0.5 sec, which excluded our non-inferiority margin of five seconds. Therefore, intubation with the AuraGain does not require more time than that with the Guedel tube. There was no difference in the first attempts to intubate between the Guedel tube and AuraGain. Our data show a slightly higher first-time intubation success rate compared with the 85% success rate achieved by Heidegger et al.,21 which could be due to the smaller patient cohorts in our study as well as the high degree of expertise of our anesthesiologists. In general, any second attempts needed for either the Guedel tube or AuraGain were because of the impaired view of the vocal cords due to excessive saliva. After suctioning the saliva, intubation was completed successfully during the second attempt. Intubation could not be completed through the AuraGain in one patient, even though the bronchoscope could be advanced easily through the vocal cords into the trachea. “Hanging-up” of the ETT prevented consequent insertion of the tube, a phenomenon described by Emmerich and Tiesmeier.22 This could occur due to a steeper entrance into the trachea compared with the flat angle of the armoured tracheal tube out of the AuraGain (Fig. 2, placement of an endotracheal tube through an AuraGain laryngeal mask device). A “too large” gap between the ETT and bronchoscope can make the passage of the ETT into the trachea impossible due to the ETT hanging on tracheal structures.23 In our study, we chose a flexible bronchoscope with a 4.8-mm outer diameter and an ETT with a 7-mm internal diameter to keep the gap between the ETT and the flexible bronchoscope similar to that in previous studies. These sizes were chosen to minimize a higher rate of second intubation attempts due to the higher gap, even if we would expect an easier passage of a smaller flexible bronchoscope through the AuraGain.21
We noted very short bronchoscopic intubation times compared with a previously reported study, which could be related to different measurement methods. Ovassapian et al. reported a three to five minute period for bronchoscopic intubation, where attaching the ETT to the ventilator and controlling capnography curves were included.24 We measured the time from entering either the Guedel tube or AuraGain with the bronchoscope until its exit once the ETT was visually confirmed to be in its correct position. It took no longer than 20 sec to instil respiration after the bronchoscope exited the ETT and to confirm respiratory activity with an adequate capnography curve, which makes our total intubation times less than one minute.
Adverse events are still common in airway management, with up to an 18% incidence in difficult intubation for elective surgery.25 When difficulties with regular intubation arise, there is the need for a plan B (i.e., an emergency pathway for difficult intubation).6 Bronchoscopic awake intubation remains the golden standard for expected difficult intubation,11 whereas the primary goal in unexpected difficult airway should be maintenance of oxygenation and limiting the number of intubation attempts in an emergency.6 The use of a second-generation supraglottic airway can be advantageous in the case of an unexpected difficult airway as well as for difficult or failed bronchoscopic intubation, and this approach should be considered in these circumstances.22 Many supraglottic airway devices were brought on the market to serve as a bridge for endotracheal intubation.26 Second-generation supraglottic airway devices are described to facilitate endotracheal intubation, even though their application can be technically challenging and efficacy data are limited.6,27 Guided intubation over a supraglottic airway remains beneficial when compared with blind intubation techniques, although the review by Caponas highlighted a 96% success rate in blind intubations using the intubating laryngeal mask airway (ILMA™).28 No recommendation can be made for blind insertion through a supraglottic airway device because of the chance of esophageal intubation, which is up to 5% in the literature,29 and the possibility of harming anatomical structures, enhancing bleeding, and making the intubation procedure more difficult.30 So as not to harm any anatomical structures, we do not recommend attempting intubation through a supraglottic airway when the Brimacombe score is 1, which is akin to blind insertion of an endotracheal tube.
This is a novel study using the Brimacombe score to evaluate the position of the slit Guedel tube. Since the slit Guedel tube is not advanced as far into the hypopharynx as a supraglottic airway, a slightly different view on the glottis is expected. We found a slightly higher number of Guedel tube patients with a Brimacombe score of 2 (i.e., vocal cords plus anterior epiglottis seen) compared with the AuraGain group due to the higher distance to the glottis.
Bronchoscope experience is needed due to the restricted length when mounting an armoured ETT with an internal diameter of 7 mm. A short part of the bronchoscope will pass the AuraGain (Fig. 2) and enter the trachea. Some skills are needed to keep the bronchoscope in the trachea while railroading the ETT through the AuraGain. The examiner must be very careful that the bronchoscope does not slide out of the trachea. If smaller (and shorter) ETT sizes are chosen, this problem can be avoided, as noted by Heidegger et al. 21
There was no significant difference in postoperative airway morbidity between the two groups. We expected slightly more reported hoarseness in the AuraGain group than in the Guedel tube patients because of the additional volume of the AuraGain, and possibly the additional fixation and traction forces of the ETT. Yet there was no difference in this parameter 24 hr after surgery.
This study has some limitations that should be considered when interpreting the results. First, all intubations were performed by three experienced anesthesiologists, and therefore, the results may not apply to less experienced physicians. Second, all patients were categorized with a non-difficult airway. As the evidence for the learning curve in regular bronchoscopic laryngoscopy is within ten intubations, learning to intubate with a bronchoscope through a supraglottic airway is a requirement.12,31 We concur with Heidegger et al. that fewer unexpected difficult airways are likely to arise by having a broad indication for bronchoscopic intubation and defining more situations as difficult.32 Further, as mentioned above, the Brimacombe score, a scale for use with the flexible bronchoscope to grade the glottis view, has not been validated for use with the Guedel device but only for a laryngeal mask airway device. Nevertheless, due to the absence of another evaluation tool, we used the Brimacombe score for both supraglottic devices, the Guedel tube and the AuraGain laryngeal mask.
Our study showed that bronchoscopic intubation with the AuraGain laryngeal mask can be performed at least as fast as regular bronchoscopic intubation and may present a good alternative in an unexpected difficult airway. Further studies are required to evaluate the AuraGain in expected difficult airways before it can be considered as a standard alternative for an unexpected difficult airway.
References
Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology 2005; 103: 429-37.
Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013; 118: 251-70.
Kleine-Brueggeney M, Theiler L, Urwyler N, Vogt A, Greif R. Randomized trial comparing the i-gel™ and Magill tracheal tube with the single-use ILMA™ and ILMA™ tracheal tube for fibreoptic-guided intubation in anaesthetized patients with a predicted difficult airway. Br J Anaesth 2011; 107: 251-7.
Langeron O, Semjen F, Bourgain JL, Marsac A, Cros AM. Comparison of the intubating laryngeal mask airway with the fiberoptic intubation in anticipated difficult airway management. Anesthesiology 2001; 94: 968-72.
American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003; 98: 1269-77.
Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 2015; 115: 827-48.
Ahmad I, Bailey CR. Time to abandon awake fibreoptic intubation? Anaesthesia 2016; 71: 12-6.
Bhat R, Mane RS, Patil MC, Suresh SN. Fiberoptic intubation through laryngeal mask airway for management of difficult airway in a child with Klippel-Feil syndrome. Saudi J Anaesth 2014; 8: 412-4.
Lee JJ, Lim BG, Lee MK, Kong MH, Kim KJ, Lee JY. Fiberoptic intubation through a laryngeal mask airway as a management of difficult airwary due to the fusion of the entire cervical spine - A report of two cases. Korean J Anesthesiol 2012; 62: 272-6.
Heidegger T, Gerig HJ, Ulrich B, Kreienbuhl G. Validation of a simple algorithm for tracheal intubation: daily practice is the key to success in emergencies–an analysis of 13,248 intubations. Anesth Analg 2001; 92: 517-22.
Schenk A, Markus CK, Kranke P. Awake fiberoptic intubation - gold standard for the anticipated difficult airway (German). Anasthesiol Intensivmed Notfallmed Schmerzther 2014; 49: 92-9.
Collins SR, Blank RS. Fiberoptic intubation: an overview and update. Respir Care 2014; 59: 865-78; discussion 878-80.
Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869.
Ryan P. RALLOC: Stata module to design randomized controlled trials. 2011. Available from URL: http://econpapers.repec.org/software/bocbocode/s319901.htm (accessed May 2017).
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377-81.
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia - revised edition 2016. Can J Anesth 2016; 63: 86-112.
Kim SH, Kim JE, Kim YH, et al. An assessment of oropharyngeal airway position using a fibreoptic bronchoscope. Anaesthesia 2014; 69: 53-7.
Blair EJ, Mihai R, Cook TM. Tracheal intubation via the Classic and Proseal laryngeal mask airways: a manikin study using the Aintree Intubating Catheter. Anaesthesia 2007; 62: 385-7.
Brimacombe J, Berry A. A proposed fiber-optic scoring system to standardize the assessment of laryngeal mask airway position. Anesth Analg 1993; 76: 457.
Dhand NK. Aine statistical calculator. Sample SizeCalculator for Comparing Two independent Means; 2014. Available from URL: http://statulator.com/SampleSize/ss2M.html (accessed May 2017).
Heidegger T, Gerig HJ, Ulrich B, Schnider TW. Structure and process quality illustrated by fibreoptic intubation: analysis of 1612 cases. Anaesthesia 2003; 58: 734-9.
Emmerich M, Tiesmeier J. The I-gel supraglottic airway: a useful tool in case of difficult fiberoptic intubation. Minerva anestesiologica 2012; 78: 1169-70.
Hakala P, Randell T. Comparison between two fibrescopes with different diameter insertion cords for fibreoptic intubation. Anaesthesia 1995; 50: 735-7.
Ovassapian A, Glassenberg R, Randel GI, Klock A, Mesnick PS, Klafta JM. The unexpected difficult airway and lingual tonsil hyperplasia: a case series and a review of the literature. Anesthesiology 2002; 97: 124-32.
Benumof JL. Management of the difficult airway. Ann Acad Med Singapore 1994; 23: 589-91.
Metterlein T, Plank C, Sinner B, Bundscherer A, Graf BM, Roth G. A comparison of fiberoptical guided tracheal intubation via laryngeal mask and laryngeal tube. Saudi J Anaesth 2015; 9: 37-41.
Darlong V, Biyani G, Baidya DK, Pandey R, Punj J. Air-Q blocker: a novel supraglottic airway device for patients with difficult airway and risk of aspiration. J Anaesthesiol Clin Pharmacol 2014; 30: 589-90.
Caponas G. Intubating laryngeal mask airway. Anaesth Intensive Care 2002; 30: 551-69.
Dimitriou V, Voyagis GS. Blind intubation via the ILMA: what about accidental oesophageal intubation? Br J Anaesth 1999; 82: 478-9.
Campbell RL, Biddle C, Assudmi N, Campbell JR, Hotchkiss M. Fiberoptic assessment of laryngeal mask airway placement: blind insertion versus direct visual epiglottoscopy. J Oral Maxillofac Surg 2004; 62: 1108-13.
Johnson C, Roberts JT. Clinical competence in the performance of fiberoptic laryngoscopy and endotracheal intubation: a study of resident instruction. J Clin Anesth 1989; 1: 344-9.
Heidegger T, Starzyk L, Villiger CR, et al. Fiberoptic intubation and laryngeal morbidity: a randomized controlled trial. Anesthesiology 2007; 107: 585-90.
Acknowledgements
The authors sincerely thank D. Rickenbacher MSc for the support in planning the trial, including submission to the Ethics Committee. We are also grateful to M. Wilhelmi PhD for copy-editing this manuscript. The Schulthess Clinic provided support for this research.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Associate Editor, Canadian Journal of Anesthesia.
Author contributions
Berthold Moser contributed substantially to the study concept. Berthold Moser, Christian Keller, and Heinz R. Bruppacher contributed substantially to the design of the study. Berthold Moser, Laurent Audigé, Christian Keller, and Heinz R. Bruppacher contributed substantially to the acquisition, analysis, and interpretation of data. Berthold Moser, Josef Brimacombe, Lukas Gasteiger, and Heinz R. Bruppacher contributed substantially to developing the methodology. Berthold Moser, Laurent Audigé, Josef Brimacombe, Lukas Gasteiger, and Heinz R. Bruppacher contributed substantially to drafting of article. Laurent Audigé, Josef Brimacombe, and Lukas Gasteiger contributed substantially to critical revision of the manuscript.
Financial disclaimer
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moser, B., Audigé, L., Keller, C. et al. Flexible bronchoscopic intubation through the AuraGain™ laryngeal mask versus a slit Guedel tube: a non-inferiority randomized-controlled trial. Can J Anesth/J Can Anesth 64, 1119–1128 (2017). https://doi.org/10.1007/s12630-017-0936-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0936-7