Abstract
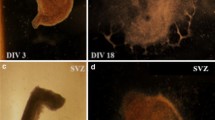
The hypothesis of enhanced vulnerability following perinatal asphyxia was investigated with a protocol combining in vivo and in vitro experiments. Asphyxia-exposed (AS) (by 21 min water immersion of foetuses containing uterine horns) and caesarean-delivered control (CS) rat neonates were used at P2-3 for preparing triple organotypic cultures (substantia nigra, neostriatum and neocortex). At DIV 18, cultures were exposed to different concentrations of H2O2 (0.25–45 mM), added to the culture medium for 18 h. After a 48-h recovery period, the cultures were either assessed for cell viability or for neurochemical phenotype by confocal microscopy. Energy metabolism (ADP/ATP ratio), oxidative stress (GSH/GSSG) and a modified ferric reducing/antioxidant power assay were applied to homogenates of parallel culture series. In CS cultures, the number of dying cells was similar in substantia nigra, neostriatum and neocortex, but it was several times increased in AS cultures evaluated under the same conditions. A H2O2 challenge led to a concentration-dependent increase in cell death (>fourfold after 0.25 mM of H2O2) in CS cultures. In AS cultures, a significant increase in cell death was only observed after 0.5 mM of H2O2. At higher than 1 mM of H2O2 (up to 45 mM), cell death increased several times in all cultures, but the effect was still more prominent in CS than in AS cultures. The cell phenotype of dying/alive cells was investigated in formalin-fixed cultures exposed to 0 or 1 mM of H2O2, co-labelling for TUNEL (apoptosis), MAP-2 (neuronal phenotype), GFAP (astroglial phenotype) and TH (tyrosine hydroxylase; for dopamine phenotype), counterstaining for DAPI (nuclear staining), also evaluating the effect of a single dose of nicotinamide (0.8 nmol/kg, i.p. injected in 100 μL, 60 min after delivery). Perinatal asphyxia produced a significant increase in the number of DAPI/TUNEL cells/mm3, in substantia nigra and neostriatum. One millimolar of H202 increased the number of DAPI/TUNEL cells/mm3 by ≈twofold in all regions of CS and AS cultures, an effect that was prevented by neonatal nicotinamide treatment. In substantia nigra, the number of MAP-2/TH-positive cells/mm3 was decreased in AS compared to CS cultures, also by 1 mM of H202, both in CS and AS cultures, prevented by nicotinamide. In agreement, the number of MAP-2/TUNEL-positive cells/mm3 was increased by 1 mM H2O2, both in CS (twofold) and AS (threefold) cultures, prevented by nicotinamide. The number of MAP-2/TH/TUNEL-positive cells/mm3 was only increased in CS (>threefold), but not in AS (1.3-fold) cultures. No TH labelling was observed in neostriatum, but 1 mM of H2O2 produced a strong increase in the number of MAP-2/TUNEL-positive cells/mm3, both in CS (>2.9-fold) and AS (>fourfold), decreased by nicotinamide. In neocortex, H2O2 increased the number of MAP-2/TUNEL-positive cells/mm3, both in CS and AS cultures (≈threefold), decreased by nicotinamide. The ADP/ATP ratio was increased in AS culture homogenates (>sixfold), compared to CS homogenates, increased by 1 mM of H202, both in CS and AS homogenates. The GSH/GSSG ratio was significantly decreased in AS, compared to CS cultures. One millimolar of H2O2 decreased that ratio in CS and AS homogenates. The present results demonstrate that perinatal asphyxia induces long-term changes in metabolic pathways related to energy and oxidative stress, priming cell vulnerability with both neuronal and glial phenotype. The observed effects were region dependent, being the substantia nigra particularly prone to cell death. Nicotinamide administration in vivo prevented the deleterious effects observed after perinatal asphyxia in vitro, a suitable pharmacological strategy against the deleterious consequences of perinatal asphyxia.





Similar content being viewed by others
Abbreviations
- ADP:
-
Adenosine diphosphate
- AIF:
-
Apoptosis-inducing factor
- AM:
-
Calcein-acetoxymethyl ester
- AS:
-
Asphyxia-exposed rats
- ATP:
-
Adenosine triphosphate
- BCA:
-
Bicinchoninic acid
- Bcl-2:
-
B-cell lymphoma 2
- Bnip3:
-
BCL2 interacting protein 3
- CS:
-
Caesarean-delivered rats
- Cx:
-
Neocortex
- DAPI:
-
4′6-Diamidino-2-phenylindole
- DIV:
-
Days in vitro
- DMEM:
-
Dulbecco’s modified Eagle medium
- DTNB:
-
5,5′-Dithiobis-2-nitrobenzoic acid
- EthD-1:
-
Ethidium-homodimer
- G:
-
Gestation day
- GFAP:
-
Glial fibrillary acidic protein
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- HIF-1α:
-
Hypoxia induced factor-1α
- i.p.:
-
Intraperitoneal
- MAP-2:
-
Microtubule-associated protein-2
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NAM:
-
Nicotinamide, niacinamide, vitamine B3
- Nix:
-
BCL2/adenovirus E1B interacting protein 3-like
- NMDA:
-
N-methyl-d-aspartate
- nNOS:
-
Neuronal nitric oxide synthase
- NO:
-
Nitric oxide
- Noxa:
-
Phorbol-12-myristate-13-acetate-induced protein 1
- PA:
-
Perinatal asphyxia
- PARP-1:
-
Poly(ADP-ribose) polymerase-1
- PBS:
-
Phosphate-buffered saline
- PLS:
-
Partial least squares
- RFU:
-
Relative fluorescence units
- ROS:
-
Reactive oxidative species
- RNS:
-
Reactive nitrosylated species
- SN:
-
Substantia nigra
- Str:
-
Neostriatum
- TH:
-
Tyrosine hydroxylase
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labelling
References
Allende-Castro C, Espina-Marchant P, Bustamante D, Rojas-Mancilla E, Neira T, Gutierrez-Hernandez MA, Esmar D, Valdes JL, Morales P, Gebicke-Haerter PJ, Herrera-Marschitz M (2012) Further studies on the hypothesis of PARP-1 inhibition as strategy for lessening the long-term effects produced by perinatal asphyxia: effects of nicotinamide and theophylline on PARP-1 activity in brain and peripheral tissue. Neurotox Res 22:79–90
Amoroso S, Tortiglione A, Secondo A, Catalano A, Montagni S, Di Renzo G, Annunziato L (2000) Sodium nitroprusside prevents chemical hypoxia-induced cell death through iron ions stimulating the activity of the Na+-Ca2+ exchanger in C6 glioma cells. J Neurochem 74:1505–1513
Ara J, Fekete S, Frank M, Golden JA, Pleasure D, Valencia I (2011) Hypoxic-preconditioning induces neuroprotection against hypoxia-ischemia in newborn piglet brain. Neurobiol Dis 43:473–485
Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E (2013) Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38:1698–1708
Bai J-Z, Lipski J (2010) Diffrential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 31:204–214
Bai P, Cantó C, Oudart H, Bruyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper R, Schoonjans K, Schreiber V, Sauve A, Menissier-de Murcia J, Auwerx J (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13:461–468.
Berger NA (1985) Poly (ADP-ribose) in the cellular response to DNA damage. Radiat Res 1001:4–15
Boksa P, Krishnamurthy A, Brooks W (1995) Effects of a period of asphyxia during birth on spatial learning in the rat. Pediatr Res 37:489–496
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad USA 1:97 (16):9082–9087.
Burke RE, Macaya A, DeVivo D, Kenyon N, Janec EM (1992) Neonatal hypoxic-ischemic or excitotoxic striatal injury results in a decreased adult number of substantia nigra neurons. Neuroscience 50:559–569
Carloni S, Carnevali A, Cimino M, Balduini W (2008) Extended role of necrotic cll death after hypoxia-ischemia-induced neurodegeneration in the neonatal rat. J Pineal Res 44:157–164
Chang S, Jiang X, Zhao C, Ferriero DM (2008) Exogenous low dose hydrogen peroxide increases hypoxia-inducible factor-1alpha protein expression and induces preconditioning protection against ischemia in primary cortical neurons. Neurosci Lett 441:134–138
Chen Y, Engidawork E, Loidl F, Dell’Anna E, Gony M, Lubec G, Andersson K, Herrera-Marschitz M (1997) Short- and long-term effects of perinatal asphyxia on monoamine, amino acids and glycolysis product levels measured in the basal ganglia of the rat. Dev Brain Res 104:19–30
Chen L, Liu L, Yin J, Luo Y, Huang S (2009) Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2-A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol 41:1284–1295
Chong ZZ, Lin S-H, Maiese K (2004) The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cer Blood Flow Metab 24:728–743
Crowley LC, Marfell BJ, Waterhouse NJ (2016) Detection of DNA fragmentation in apoptotic cells by TUNEL. Cold Spring Harb Protoc; doi 10:1101
De Torres C, Munell F, Reventos J, Macaya A (1997) Identification of necrotic cell death by TUNEL assay in the hypoxic-ischemic neonatal brain. Neurosci Lett 230:1–4
Dell’Anna E, Chen Y, Loidl F, Andersson K, Luthman J, Goiny M, Rawal R, Lindgren T, Herrera-Marschitz M (1995) Short-term effects of perinatal asphyxia studied with Fos-immunocytochemistry and in vivo microdialysis in the rat. Exp Neurol 131:279–287
Dell’Anna E, Chen Y, Engidawork E, Andersson K, Lubec G, Luthman J, Herrera-Marschitz M (1997) Delayed neuronal death following perinatal asphyxia in rat. Exp Brain Res 115:105–115
Deng W (2010) Neurobiology of injury to the developing brain. Nat Dev Neurology 6:328–336
Desagher S, Glowinski J, Premont J (1996) Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci 16:2553–2562
Desagher S, Glowinski J, Premont J (1997) Pyruvate protects neurons against hydrogen peroxide-induce toxicity. J Neurosci 17:9060–9067
Douglas-Escobar M, Weiss MD (2015) Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr 169:397–403.
Duchen MR, Leyssens A, Crompton M (1998) Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol 142:975–988
Edwards A, Mehmet H (2008) Apoptosis in perinatal hypoxic-ischaemic cerebral damage. Neuropathol Appl Neurobiol 22(6):494–498
Engidawork E, Chen Y, Dell’Anna E, Goiny M, Lubec G, Andersson K, Herrera-Marschitz M (1997) Effects of perinatal asphyxia on systemic and intracerebral glycolysis metabolism and pH in the rat. Exp Neurol 145:390–396
Ezquer ME, Valdez SR, Seltzer AM (2006) Inflammatory responses of the substantia nigra after acute hypoxia in neonatal rats. Exp Neurol 197:391–398
Feeney CJ, Frantseva MV, Carlen PL, Pennefather PS, Shulyakova N, Shniffr C, Mills LR (2008) Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res 1198:1–15
Ferriero DM (2001) Oxidant mechanisms in neonatal hypoxia-ischemia. Dev Neurosci 23:198–202
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Gidday JM (2006) Cerebral preconditioning and ischemic tolerance. Nature Rev 7:437
Gilland E, Puka-Sundvall M, Hillered L, Hagberg H (1998) Mitochondrial function and energy metabolism after hypoxia-ischemia in the immature rat brain: involvement of NMDA-receptors. J Cer Blood Flow Metab 18:297–304
Gomez-Urquijo SM, Hokfelt T, Ubink R, Lubec G, Herrera-Marschitz M (1999) Neurocircuitries of the basal ganglia studied in organotypic cultures: focus on tyrosine hydroxylase, nitric oxide synthase and neuropeptide immunocytochemistry. Neuroscience 94:1133–1151
Gonzalez-Flores A, Aguilar-Quesada R, Siles E, Pozo S, Rodriguez-Lara MI, Lopez-Jimenez L, Lopez-Rodriguez M, Peralta-Leal A, Villar D, Martin-Oliva D, del Peso L, Berra E, Oliver FJ (2014) Interaction between PARP-1 and HIF-2alpha in the hypoxic response. Oncogene 33:891–898
Griffiths EJ, Halestrap AP (1993) Protection by cyclosporine A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol 25:1461–1469
Hagberg H, Edwards AD, Groenendaal F (2016) Perinatal brain damage: the term infant. Neurobiol Dis 92:102–112
Herrera-Marschitz M, Ungerstedt U (1984) Evidence that apomorphine and pergolide induce rotation in rats by different actions on D1 and D3 receptor sites. Eur J Pharmacol 98:165–176
Herrera-Marschitz M, Morales P, Leyton L, Bustamante D, Klawitter V, Espina-Marchant P, Allende C, Lisboa F, Cunich G, Jara-Cavieres A, Neira T, Gutierrez-Hernandez MA, Gonzalez-Lira V, Simola N, Schmitt A, Morelli M, Andrew Tasker R, Gebicke-Haerter PJ (2011) Perinatal asphyxia: current status and approaches towards neuroprotective strategies, with focus on sentinel proteins. Neurotox Res 19:603–627
Herrera-Marschitz M, Neira-Pena T, Rojas-Mancilla E, Espina-Marchant P, Esmar D, Perez R, Munoz V, Gutierrez-Hernandez MA, Rivera B, Simola N, Bustamante D, Morales P, Gebicke-Haerter PJ (2014) Perinatal asphyxia: CNS development and deficits with delayed onset. Front Neurosci 8:1–1
Hoeger H, Engelmann M, Bernet G, Seidl R, Bubna-Littitz H, Mosgoeller W, Lubec B, Lubec G (2000) Long term neurological and behavioral effects of graded perinatal asphyxia in the rat. Life Sci 66:947–962
Hong SJ, Dawson TM, Dawson VL (2004) Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signalling. TIPS 25:259–264
Hwang J-J, Choi S-Y, Koh J-Y (2002) The role of NADPH oxidase, neuronal nitric oxide synthase and poly(ADP ribose) polymerase in oxidative neuronal death induced in cortical cultures by brain-derived neurotrophic factor and neurotrophin-4/5. J Neurochem 82:894–902
Ikeda T, Mishima K, Yoshikawa T, Iwasaki K, Fuijiwara M, Xia YX, Ikenoue T (2001) Selective and long-term learning impairment following neonatal hypoxic-ischemic brain insult in rats. Behav Brain Res 118:17–25
Jendrach M, Mai S, Pohl S, Voeth M, Bereiter-Hahn J (2008) Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mithochondrion 8:293–304
Jiang X, Mu D, Manabat C, Koshy AA, Christen S, Tauber MG, Vexler ZS, Ferreiro DM (2004) Different vulnerability of immature murine neurons to oxygen-glucose deprivation. Exp Neurol 190:224–232
Johnston M, Hoon A (2000) Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J Child Neurol 15(9):588–591
Kauppinen TM, Swanson RA (2007) The role of poly(ADP-ribose) polymerase-1 in CNS disease. Neurosci 147:1267–1272
Kauppinen TM, Suh SW, Higashi Y, Berman AE, Escartin C, Won SJ, Wang C, Cho SH, Gan L, Swanson RA (2011) Poly(ADP-ribose)polymerase-1 modulates microglial esponses to amyloid B. J Neuroinflammation 8:152
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70:1469–14680
Klawitter V, Morales P, Johansson S, Bustamante D, Goiny M, Gross J, Luthman J, Herrera-Marschitz M (2005) Effect of perinatal asphyxia on cell survival, neuronal phenotype and neurite growth evaluated with organotypic triple cultures. Amino Acids 28:149–155
Klawitter V, Morales P, Bustamante D, Gomez-Urquijo S, Hökfelt T, Herrera-Marschitz M (2007) Neuronal plasticity of basal ganglia following perinatal asphyxia: neuroprotection by nicotinamide. Exp Brain Res 180:139–152
Krasnikov BF, Kuzminova AE, Zorov DB (1997) The Ca2+-induced pore opening in mitochondria energized by succinate-ferricyanide electron transport. FEBS Lett 419:137–140
Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Bramilla E, Negoescu A (1998) TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J Histochem Cytochem 46:327–334
Low JA (2004) Determining the contribution of asphyxia to brain damage in the neonate. J Obstet Gynaecol Res 30:276–286
Luo X, Kraus WL (2011) On PAR with PARP: cellular stress signaling through poly (ADP-ribose) and PARP-1. Genes Dev 26:417–432
Marriott AL, Rojas-Mancilla E, Morales P, Herrera-Marschitz M, Tasker RA (2016) Models of progressive neurological dysfunction originating early in life. Prog Neurobiol. doi:10.1016/j.pneurobio.2015.10.001
Martin-Oliva D, Aguilar R, Ovalle F, Muñoz J, Martinez R, García del Moral R, Ruiz J, Villuendas R, Piris M, Oliver F (2006) Inhibition of poly(ADP-ribose) polymerase modulates tumor-related gene expression, including hypoxia inducible factor-1 activation during skin carcinogenesis. Cancer Res 66(11):5744–5766
Mattson M (2007) Mitochondrial regulation of neuronal plasticity. Neurochem Res 32(4–5):707–715
Matyash V, Kettenmann H (2010) Heterogeneity in astrocyte morphology and physiology. Brain Res Rev 63:2–10
McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM (2003) Selective vulnerability of subplate neurons after early neonatal hypoxi-ischemia. J Neurosci 23:3308–3315
Mischel RE, Kim YS, Sheldon RA, Ferriro DM (1997) Hydrogen peroxide is selectively toxic to immature murine neurons in vitro. Neurosci Lett 231:17–20
Moncada S, Bolaños J (2006) Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem 97(6):1676–1689
Morales P, Reyes P, Klawitter V, Huaiquín P, Bustamante D, Fiedler J, Herrera-Marschitz M (2005) Effects of perinatal asphyxia on cell proliferation and neuronal phenotype evaluated with organotypic hippocampal cultures. Neuroscience 135:421–431
Morales P, Fiedler JL, Andres S, Berrios C, Huaiquin P, Bustamante D, Cardenas S, Parra E, Herrera-Marschitz M (2008) Plasticity of hippocampus following perinatal asphyxia: effects on postnatal apoptosis and neurogenesis. J Neurosci Res 86:2650–2662
Morales P, Simola N, Bustamante D, Lisboa F, Fiedler J, Gebicke-Haerter P, Morelli M, Tasker RA, Herrera-Marschitz M (2010) Nicotinamide prevents the effect of perinatal asphyxia on apoptosis, non-spatial working memory and anxiety in rats. Exp Brain Res 202:1–14
Mukherjee SK, Klidman LK, Yasharel R, Adams JD Jr (1997) Increased brain NAD prevents neuronal apoptosis in vivo. Eur J Pharmacol 330:27–34
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. TINS 26(10):523–530
Neira-Peña T, Rojas-Mancilla E, Munoz-Vio V, Perez R, Gutierrez-Hernandez M, Bustamante D, Morales P, Hermoso MA, Gebicke-Haerter P, Herrera-Marschitz M (2015) Perinatal asphyxia leads to PARP-1 overactivity, p65 translocation, IL-1β and TNF-α overexpression, and apoptotic-like cell death in mesencephalon of neonatal rats: prevention by systemic neonatal nicotinamide administration. Neurotox Res 27(4):453–465
Northington FJ, Graham EM, Martin LJ (2005) Apotosis in perinatal hypoxic-ischemic brain injury: how important is it and should it be inhibited? Brain Res Rev 50:244–257
Orrenius S, Nicotera P, Zhivotovsky B (2011) Cell death mechanisms and their implications in toxicology. Toxicol Sci 119:3–19
Østergaard K, Zimmer F (1995) Organotypic slice cultures of the rat striatum: an immunocytochemical, histochemical and in situ hybridization study of somatostatin, neuropeptide Y, nicotinamide adenine dinucleotide phosphate-diaphorase, and enkephalin. Exp Brain Res 103(1):70–84
Palmer C, Brucklacher RM, Christensen MA, Vannucci RC (1990) Carbohydrate and energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. J Cereb Blood Flow Metab 10:227–235
Pérez-Pinzón MA, Xu GP, Born J, Lorenzo J, Busto R, Rosenthal M, Sick TJ (1999) Cytochrome C is released from mitochondria into the cytosol after cerebral anoxia or ischemia. Journal of cerebral blood flow metabolism 19(1):39–43
Pieper AA, Walles T, Wei G, Clements EE, Verma A, Snyder SH, Zweier JL (2000) Myocardial postischemic injury is reduced by polyADPribose plymerase-1 gene disruption. Mol Med 6:271–282
Plenz D, Kitai ST (1996a) Organotypic cortex-striatum-mesencephalon cultures: the nigro-striatal pathway. Neurosci Lett 209:177–180
Plenz D, Kitai ST (1996b) Generation of high frequency oscillations in cortical circuits of somatosensory cortex cultures. J Neurophysiol 76:4001–4005
Plenz D, Herrera-Marschitz M, Kitai ST (1998) Morphological organization of the globus pallidussubthalamic nucleus system studied in organotypic cultures. J Comp Neurol 397:437–457
Rehncrona S, Folbergrova J, Smith D, Siesgo B (1980) Influence of complete and pronounced incomplete cerebral ischemia and subsequent recirculation on cortical concentrations of oxidized and reduced glutathione in the rat. J Neurochem 34(3):477–486
Saito A, Maier CM, Narasimhan P, Nishi T, Song YS, Yu F, Liu J, Lee SY, Nito C, Kamada H, Dodd RL, Hsieh LB, Hassid B, Kim EE, González M, Chan PH (2005) Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol 31(1–3):105–116
Schraufstatter IU, Hyslop PA, Hinshaw DB, Spragg RG, Sklar LA, Cochrane CH (1986) Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. PNAS USA 83:4908–4912
Sies H (2017) Hydrogen peroxide as a central redox signalling molecule in physiological oxidative stress: oxidative eustress. Redox Biol 11:613–619
Skulachev VP (1996) Role of uncoupled and non-coupled oxidation in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys 29:169–202
Smith CM, Chen Y, Sullivan ML, Kochanek PM, Clarck RSB (2011) Autophagy in acute brain injury: feast, famine, or folly? Neurobiol Dis 43:52–59
Sowter HM, Ratcliffe PJ, Watson P, Greenberg HH, Harris AL (2001) HIF-dependent regulation of hypoxic induction of cell death factors BNIP3 and NIX in human tumors. Cancee Res 61:6669–6673
Ungerstedt U (1971) Stereotaxic mapping of the monoamine pathway in the rat brain. Acta Physiol Scand Suppl 367:1–48
Van de Berg WD, Schmitz C, Steinbusch HWM, Blanco CE (2002) Perinatal asphyxia induced neuronal loss by apotosis in the neonatal rat striatum: a combined TUNEL and stereological study. Exp Neurol 174:29–36
Vangeison G, Carr D, Federoff H, Rempe D (2008) The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci 28(8):1988–1993
Vannucci RC, Towfighi J, Vannucci SJ (1998) Hypoxic preconditioning and hypoxic-ischemic brain damage in the immature rat: pathologic and metabolic correlates. J Neurochem 71:1215–1220
Wallin C, Puka-Sundvall M, Hagberg H, Weber SG, Sandberg M (2000) Alterations in glutathione and amino acid concentrations after hypoxia-ischemia in the immature rat brain. Dev Brain Res 125:51–60
Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2(12):1–13
Weis SN, Toniazzo AP, Ander BP, Zhan X, Caraga M, Ashwood P, Wyse ATS, Netto CA, Sharp FR (2014) Autophagy in the brain of neonates following hypoxia-ischemia shows sex- and region-specific effects. Neurosci 256:201–209
Whittemore ER, Loo D, Cotman C, Carl W (1994) Exposure to hydrogen peroxide induces cell death via apoptosis in cultured rat cortical neurons. Neuroreport 5(12):1485–1488
Whittemore ER, Loo DT, Watt JA, Cotman CW (1995) A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience 67:921–932
Wold S, Sjostrom M, Carlson R, Lundstedt T, Hellberg S, Skagerberg B, Wikstrom C, Ohman J (1986) Multivariate design. Analytica Chim Acta 191:17–32
Xu L, Voloboueva LA, Ouyang Y, Emery J, Giffard R (2009) Overexpression of mitochondrial HSP70/HSP75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab 29:365–374
Yang J, Klaidman LK, Nalbandian A, Oliver J, Chang ML, Chan PH, Adams Jr JD (2002) The effect of nicotinamide on enrgy metabolism following transient focal cerebral ischemia in Wistar rats. Neurosci Lett 333:91–94
Yin W, Signore AP, Iwai N, Cao G, Gao Y, Chen J (2008) Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39:3057–3063
Yue X, Mehmet H, Penrice J, Cooper C, Cady E, Wyatt JS, Reynolds EOR, Edwards AD, Squie MV (1997) Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropath Appl Neurobiol 23:16–25
Acknowledgements
We would like to than the following contract grant sponsors: FONDECYT-Chile; Millenium Institute Initiative (BNI P09-015-F) and MHMarschitz Foundation, Sweden. RPL (#21130739), CLR (#21140281), ATB (#21151232) and EP (#21171433) are CONICYT-Chile fellows. VVM was a MECESUP-Chile fellow (UCH0704).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest on any section of the manuscript.
Rights and permissions
About this article
Cite this article
Perez-Lobos, R., Lespay-Rebolledo, C., Tapia-Bustos, A. et al. Vulnerability to a Metabolic Challenge Following Perinatal Asphyxia Evaluated by Organotypic Cultures: Neonatal Nicotinamide Treatment. Neurotox Res 32, 426–443 (2017). https://doi.org/10.1007/s12640-017-9755-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9755-4