Abstract
Purpose
The conversion of broiler waste to activated carbon results in a significant ash fraction that contains several species thought to be responsible for the adsorption capabilities of these carbons. The objectives of this study were to determine (1) the effects of acid washing, a typical regeneration method on the ability of these carbons to adsorb metal cations, (2) the extent to which select cations and anions are released from poultry waste carbons under conditions of varying acid strength, and (3) which of the elements in the ash fraction may contribute to ion binding.
Methods
Litter and cake activated carbons were placed in solutions of hydrochloric acid at concentrations ranging from 0.05 to 2.0 M for 30 min to 4 h. The resulting solutions were analyzed for the release of the seven select cations and anions. The carbons were then evaluated for their ability to adsorb copper to determine the impact of acid washing on copper adsorption.
Results
Calcium, phosphorus and magnesium were released in the greatest concentrations (1.7–2.1, 1.6–2.5, and 0.7–0.8 mmol/g respectively). Of these seven species, phosphorus and sulfur were of particular interest due to their potential for binding copper ions.
Conclusions
The copper adsorption was significantly impacted by the acid washing process with a reduction in adsorption for the litter carbon from 41.96 to 0.32 mg/g and the cake carbon from 85.31 to 23.03 mg/g with an increase in acid concentration from 0.05 to 2.0 M HCl.
Similar content being viewed by others
Introduction
Currently, commercially available activated carbons are sourced from either coal or coconut shells, both of which are highly dependent upon market fluctuations. Also, activated carbons are typically employed for the removal of organic molecules, for example taste and odor compounds in drinking water. However, commercially available carbons are not known for their ability to adsorb metal cations unlike those from animal sources [1, 2]. The development of activated carbons from waste sources is not a new field, yet it is slowly emerging as one of interest to those looking for a more economical source material with effective treatment capabilities [1, 3–8]. The use of agricultural by-products such as pecan shells, rice hulls and sugar cane bagasse as alternative sources for carbon has been investigated. These carbons were assessed for the capabilities with regard to metals and organics uptake and were found to be comparable or better than commercial grade carbons from coal or coconut shells [9–12].
Significant differences between carbons from plant and animal waste lie in the surface areas of the carbons and their mechanisms of adsorption. The plant-based carbons tend to have high surface areas (500–1,500 m2/g) [13, 14] with functional groups such as carboxyl, hydroxyl, carbonyl, anhydride, ether-type, lactone, and lactal [10]. Steam activated carbons from animal-based sources such as broiler litter and cake have lower surface areas of about 350–500 m2/g [1] and little is known about their mechanism of adsorption. Previous studies by Lima and Marshall [1, 15] demonstrate copper is a good indicator for positively charged metals due to its ubiquitous nature, being present in pipes and wastewater streams in general. Other metals have previously been tested including cadmium and zinc and provided similar results [15]. Isotherms using swine manure-based carbons under similar acid wash conditions (0.1 M HCl) have been conducted with copper concentrations of 1–25 mM (7 points) and published by Lima and Marshall [12]. Qui and Guo [16] found poultry litter-based activated carbon contained high contents of ash, nitrogen, phosphorus, Cu, Zn, and As similar to the results found by Fitzmorris et al. [17].
This study was designed to better describe the mechanisms and behavior of the animal waste carbons. Due to the presence of low surface areas, it is hypothesized that the animal waste carbons rely on chemical adsorption, not physical. The surface area impacts adsorption of these positively charged metals only as it applies to access to the functional groups of interest, namely the phosphate groups. This highlights the importance of the surface chemistry of the carbons, particularly the characteristics of the ash fraction. The potential impact of regeneration of these carbons particularly as related to the removal of some of the inorganic material is investigated as related to the adsorptive capabilities of the carbons. The objectives of this study are threefold: (1) to determine the effects of acid washing, a typical regeneration method on the ability of these carbons to adsorb metal cations, (2) to quantify the release of select cations and anions from the ash fraction of broiler manure-based carbons as a function of the HCl concentration of the acid wash and (3) to determine which anion in the ash fraction is the best candidate responsible for copper ion (metal ion) binding.
Materials and Methods
Materials
The broiler litter and cake were obtained from the United States Department of Agriculture, Agriculture Research Service (USDA-ARS), Genetics and Precision Agriculture Research Unit (Mississippi State, MS, USA). The differentiation between broiler litter and broiler cake is made based on the presence of wood shavings in litter (5–30 %) and in cake (<5 %). Wood shavings are utilized as bedding material.
Drying and Pelletization
Broiler litter and broiler cake were dried to a moisture content of <10 % and milled in a Retsch cross-beater mill (Glen Mills, Clifton, NJ, USA) to a particle size of less than US 20 mesh (<1 mm). The manure was then either rehydrated or dried to obtain a moisture level of 15–25 %, determined to be ideal for efficient pelletization of this type material. Moisture content was monitored by using a Sartorius Moisture Analyzer model MA 51 (Sartorius, Brentwood, NJ, USA). The samples were pelletized in a PMCL5 Lab pellet mill (California Pellet Mill, Merrimack, NH, USA) equipped with a 5 mm die plate. The pellets produced were cylindrical with an approximate diameter of 5 mm and length of 5 mm.
Pyrolysis and Activation
Individual samples of the pelletized manure were placed in a ceramic evaporating dish and then placed in a bench furnace equipped with a retort (Lindberg/Blue M, Watertown, WI, USA). Pellets were pyrolyzed at 700 °C for 1 h under a flow of nitrogen gas set at a flow rate of 1.6 L/min. Steam activation was conducted by injecting water at a flow rate of 3 mL/min using a peristaltic pump into the flow of nitrogen gas entering the heated retort. Pyrolyzed chars were activated at 800 °C for 45 min. The optimum activation conditions were determined from previous studies using copper adsorption rates and BET surface area analysis [1]. Activated carbons were allowed to cool to room temperature overnight in the retort.
Percent ash was determined by heating 2 g samples of the carbons to 650 °C for 6 h under a flow of breathing air in a retort-equipped bench furnace. Sample weights were recorded and the heating process repeated again to ensure constant sample weights were obtained. Multipoint BET (Brunauer Emmett Teller) surface area measurements were obtained with nitrogen adsorption isotherms at 77° K using a Nova 2000 surface area analyzer (Quantachrome, Boynton Beach, FL, USA).
Prior to analysis, the carbon was milled and sieved to a particle size >2.8 mm (U.S. 7 mesh) to ensure a consistent product. Triplicate samples of the raw broiler litter and cake along with the corresponding carbons were digested with concentrated HCl to determine the elemental composition of calcium, copper, iron, magnesium, phosphorus, sulfur, and zinc (EPA Method 3051). The digested samples were analyzed by inductively coupled plasma (ICP) spectrometry using a dual view, Leeman Labs Profile ICP-AES (Leeman Labs, Hudson, NH, USA).
Release Study
In this study, the broiler litter and cake carbons were placed in varying acid concentrations ranging from 0.05 to 2.0 M HCl. For the experiments, 2.5 g of each carbon was added to 250 mL of each acid solution. The samples were stirred at 300 rpm with overhead Lab Egg stirrers (IKA) for 4 h with aliquots taken at 30 min, 1, 2, and 4 h. The pH was checked at each sample time interval. The samples were then drawn through a 0.22 μm pore size Millipore filter (Millipore Corp., Bedford, MA, USA) and analyzed with ICP for the following seven elements: phosphorus, sulfur, copper, zinc, calcium, magnesium, and iron. The analysis was conducted in triplicate. Following the 4 h samples, the carbon was filtered with a U.S. 7 mesh sieve and rinsed with deionized water for 30 s. The carbon was then dried in a vacuum oven at 80 °C for 2 h. After drying, attrition was assessed by placing 3 g of carbon (18 × 40 mesh) in a 250 mL Erlenmeyer flask, which was then continuously agitated at 200 rpm in the presence of ten 5 g glass marbles for 15 min at 25 °C. Percent attrition was measured as the ratio between the material lost by a US 40 mesh screen to the initial weight.
Adsorption Study
The carbon recovered from the release study above was placed in 20 mM CuCl2 2H2O (F.W. 170.5 g) buffered with 0.07 M sodium acetate and 0.03 M acetic acid for a pH of 4.8. The ratio of carbon to copper solution was 1:100 (0.25 g carbon: 25 mL copper solution). These samples were stirred for 24 h with overhead Lab Egg stirrers (IKA, Wilmington, NC, USA). Aliquots were drawn off in a disposable syringe and filtered through a 0.22 μm pore size Millipore filter. The samples were diluted 1:100 by volume with 2 % ultrapure nitric acid and analyzed by ICP. These samples were also conducted in triplicate.
Surface Properties
The elemental composition of litter samples was measured by energy dispersive X-ray microanalysis (EDAX, Ametek, Inc.) using an X-ray detector attached to the Environmental Scanning Electron Microscope, SEM (Philips, XL 30). The working distance was set to ~10 mm with a magnification of 2,500–50,000 X. The acceleration voltage of the electron beam was 17 kV. Samples were coated with gold/palladium (for SEM) and carbon (for EDAX) for analysis purposes.
Results
Carbon Properties
Carbons produced from broiler litter and broiler cake by steam activation are characterized by high ash content and low BET surface area (Table 1). Percent yield was higher with litter as starting material (Table 1). This observation is most likely due to the higher percentage of wood chips in the litter versus the cake. BET surface areas of the broiler litter and cake carbons (283 and 149 m2/g, respectively) are significantly lower than that of plant or coal-based carbons, which normally have surface areas of 500–1,500 m2/g [13, 14]. The ash content is considerably higher in the manure-based carbons than plant-based carbons, which is usually <10 % [18]. The high level of ash in both samples (81 % for the litter and 79 % for the cake) indicates a significant amount of inorganic material in the broiler litter and cake carbons. The major contributors to the ash fraction are calcium, phosphorus and magnesium as indicated in Table 1. Although not reported herein, ash also contains large amounts of potassium.
All of the elements determined except carbon (C) and nitrogen (N) increased in concentration as the manure starting material was converted to activated carbon. At high temperatures and in the presence of limited oxygen, elemental carbon probably was volatilized during pyrolysis and emitted principally as methane, carbon monoxide and carbon dioxide. Nitrogen was likely released as oxides of nitrogen (NOx) at the high pyrolysis temperatures. Sulfur could have been released as hydrogen sulfide and/or sulfur dioxide. The observation that this element stayed relatively stable during pyrolysis indicates that it may exist as the sulfate anion in the raw manure. Since the other elements were non-volatile, they accumulated in the carbon as the amount of carbon and nitrogen was reduced during pyrolysis.
Mineral phases and how they are distributed can be depicted with EDAX elemental maps. SEM–EDAX can provide a comprehensive picture of the organic and inorganic chemistry on a carbon sample [19] The surface analysis using the EDAX system in conjunction with the SEM provides the change in surface properties of the carbons prior to adsorption compared to after adsorption (Fig. 1). The EDAX method can provide qualitative data related to the proportionality of the elements. Comparison between before and after adsorption of copper by the carbon shows not only the expected increase in percentage of copper, but also an increase in calcium, oxygen and phosphorus. Declines were noted in carbon, chlorine and potassium. A previous study [20] using X-ray/scanning electron microscope for delineating the surface properties of char from waste sources found most particles are present as complex substances of carbon and inorganic constituents combined. These results are consistent with the results found in our study.
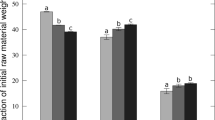
Release Study
Varying concentrations of HCl were used to affect the release of the cations calcium, magnesium, iron, copper and zinc and the anions phosphorus and sulfur from the manure-based carbons. Ion release from the carbons was monitored at different time intervals for each acid concentration. Maximum release was obtained between 2 and 4 h (data not shown). Thus, a 4 h exposure of the carbons to the acid was used in subsequent studies (Table 2). Results from the ion release studies for both broiler litter carbon (Fig. 2) and broiler cake carbon (Fig. 3) show that the dominant trend was the higher the acid concentration, the greater the release of the cations and anions. Those ions exhibiting significant release from the acid-washed carbons were calcium, magnesium, phosphorus and sulfur, since they were in the highest concentrations in the ash fraction. The calcium and magnesium levels found at the highest HCl concentration (2M) from the broiler litter carbon reached a maximum of 1.7 and 0.8 mmol/g, respectively. Calcium and magnesium levels extracted from the broiler cake carbon contained 2.1 mmol/g of calcium and 0.7 mmol/g of magnesium. The levels of phosphorus and sulfur were noted at 1.6 and 0.1 mmol/g, respectively, in the broiler litter and 2.5 and 0.1 mmol/g, respectively, in the broiler cake at the highest HCl concentration of 2M. The release of these cations and anions were a function of the extractant concentration. Ion extraction occurred at the lowest HCl concentration used (0.05 M) and appeared to reach a maximum at 1.0 M HCl. Also, there are instances in Table 2 where the percentage reported indicates a >100 % recovery of magnesium in the broiler litter carbon and calcium and phosphorus in the broiler cake activated carbon. These anomalies can be explained by the standard deviation (not reported—not determined to be statistically significant after analysis) of the samples and represent a complete recovery or release of the element in the noted acid solutions.
The likelihood of both phosphorus and sulfur occurring in the anionic form as PO4 3− and SO4 2− is high based on the chemical properties and pC-pH relationship of phosphate and sulfate [22] (Table 1). Phosphorus, especially, is present in high concentration in the ash fraction of both activated carbons and if it exists as the phosphate, it can facilitate the adsorption of cationic metals, such as copper and zinc [21, 22]. Sulfur as the sulfate anion is about 10 times lower in concentration than phosphorus and probably plays a much less significant role in metal ion adsorption [22]. Also, total surface charge was measured in these carbons [1], and it was found that they have an overall negative surface charge, which is likely attributed to their phosphate and sulfate (due to the significant presence of these two elements in the raw litter and carbon). Total surface charge was measured at 0.32 mmol H+ eq/g carbon for pH5 for broiler litter carbons activated under the same conditions as those in this study (1 h pyrolysis + 45 min activation at 800 °C with 3 mL/min FR).
Adsorption
After acid washing at each HCl concentration from 0.05 to 2.0 M, the activated carbons were examined for their ability to adsorb copper ion from a 20 mM solution of copper chloride (Table 2 and Fig. 4). For both broiler litter and broiler cake carbons, copper ion adsorption decreased with an increase in acid concentration used for washing. In a previous study, typical copper adsorption achieved by the carbons from broiler litter was 1.2 mmol/g and broiler cake was 1.9 mmol/g [1]. This study was conducted by washing carbons with 0.1 M HCl for 1 h after activation to partially remove ash material from the carbon. In contrast, the present study reported copper ion adsorption of 0.5 mmol/g for broiler litter-based carbon and 1.3 mmol/g for broiler cake-based carbon, using 0.1 M acid but a 4 h extraction period. Following acid washing for 4 h with our method, the copper adsorption ranged from 0.7 mmol/g of activated carbon using 0.05 M HCl to 0.01 mmol/g of activated carbon using 2.0 M HCl for the broiler litter activated carbon and 1.3 mmol/g using 0.05 M HCl to 0.20 mmol/g using 2.0 M HCl for the broiler cake activated carbon. The broiler cake activated carbon had greater ability to remove copper ion at all acid concentrations compared to broiler litter activated carbon. Therefore, a component of the ash fraction that is involved with copper ion binding is being removed to a certain extent by the acid wash. This component is dependent upon the concentration of acid used for the wash.
Since the presence of phosphorus in the ash fraction appears to be in a stable, anionic form, the release of phosphorus from the ash fraction was monitored and compared to copper ion adsorption in the litter-based and cake-based activated carbons. Figure 5 shows copper ion adsorbed compared to phosphorus released by washing the carbons at various HCl concentrations for the 4 h period. In both broiler litter and broiler cake activated carbons, the curves are almost mirror images of one another. An increase in phosphorus release resulted in a concomitant decrease in copper ion adsorption with a correlation factor of 0.93. Also of note is the correlation of copper adsorption to the release of calcium (0.96), copper (0.95) and iron (0.97).
The kinetics study on elements released during acid wash showed that the majority of the reactions taking place are zero order and therefore independent of concentration, and controlled mainly by the surface area available. The k-rate constants were calculated for each of the metals adsorbed by each activated carbon by the formula below [23].
where C = Final concentration, Co = Initial concentration, k = Kinetic rate constant, t = Time.
Of the elements examined, kinetic rates were highest for calcium and phosphorus at the 2.0 M acid concentration in both activated carbons examined. It is important to note that the kinetic rates were significantly lower for the litter carbons versus the cake carbons (Figs. 6 and 7).
Discussion
The results presented in this study give strong support that the presence of phosphorus in the ash fraction of broiler manure-based carbons is involved in copper ion adsorption. Of the elements evaluated in manure-based activated carbons, phosphorus and sulfur, phosphorus is present at sufficiently high concentrations to bind the amounts of copper ion observed. Sulfur, as the sulfate anion, would be too low in concentration to account for the binding levels determined. Unfortunately, as the results show, significant amounts of phosphorus are removed by an HCl wash. The removal of phosphorus is dependent on the acid concentration of the wash solution. In order to remove a metal ion such as copper ion via adsorption by an activated carbon rich in phosphorus, the phosphorus should be in the anionic form. However, the form (orthophosphate, polyphosphate) of phosphorus in the ash fraction is not known. Since phosphorus originates in the manure, it is present as both organic (phytic acid) and inorganic phosphate in the animal’s feed. However, when heated to the high temperatures (700–800 °C) required to produce an activated carbon, phosphorus may change to another form, such as a polymer or polyphosphate. Additionally, the phosphorus may react with organic constituents in the manure. Regardless of its form, exposure to increasing concentrations of HCl releases it from the carbon, thus reducing the copper ion binding capabilities of the carbon. This release could be simple acid hydrolysis of the bound phosphorus, thus solubilizing the phosphorus in the acid wash (Figs. 8, 9).
In most commercial activated carbons, adsorption is due to available surface area and not so heavily dependent upon the functionality of the surface groups. In addition, commercial carbons are generally used for the adsorption of organics, and not metal ions. It is evident by this study that the carbons from broiler manure are different. If adsorption was due to surface area alone, the adsorption capacity of broiler manure carbons should have increased following acid washing with the removal of ash and various other inorganic materials from the surface of the carbons, thereby creating more available surface sites for the binding of the copper ions [24]. However, the opposite occurred and metal ion adsorption by the activated carbons significantly decreased following the acid wash procedure. The ionized form of the phosphorus in the functional groups on the carbon is hypothesized to be phosphate (PO −34 ) which would bind the positively charged metals of interest, in this case copper. At pH of 5, the pH of peak adsorption as determined by previous studies [17], the phosphorus should be in the form of dihydrogen phosphate (H2PO4 − 99.3 %). This peak adsorption represents the balance between the metals being available in solution (pH is below the precipitation point) and the phosphorus being in a negatively charged form (pH is high enough to have the dihydrogen phosphate form instead of the phosphoric acid form). Since, phosphorus is most likely involved in the mechanism for copper ion adsorption, selective removal of other anions and cations from the ash fraction while retaining the phosphorus content would have been preferred. Unfortunately, this is not the case. In order to retain copper ion adsorption in these carbons, conditions under which the carbons are used must retain phosphorus. Most activated carbon applications are conducted at a pH at or around neutrality, and at that pH value only small amounts of anions similar to phosphorus, namely, arsenate and selenate, were released from broiler manure-based carbons [17]. Thus, phosphorus may be stable and not extracted at pH 7.
Conclusions
-
Subjecting broiler litter- and broiler cake-based carbons to an acid wash with HCl, removes constituents of the ash fraction based on the concentration of the acid used for the wash.
-
Removal of a major ash component, phosphorus, correlates well with a decrease in copper ion adsorption.
-
Considering that phosphorus is likely present as an anion in the ash fraction, and that it is present in relatively high concentrations, it may be responsible for binding copper ions from solution. Therefore, the mechanism of metal ion binding in animal waste carbons may be quite different from that of plant waste carbons where the presence of carbon-oxygen-containing functional groups may contribute to metal ion binding.
References
Lima, I.M., Marshall, W.E.: Granular activated carbons from broiler manure: physical, chemical and adsorptive properties. Bioresour Technol. 96, 699–706 (2005)
Guo, M., Qiu, G., Song, W.: Poultry litter-based activated carbon for removing heavy metal ions in water. Waste Manag. 30, 308–315 (2010)
Chiang, P.C., You, J.H.: Use of sewage sludge for manufacturing adsorbents. Can. J. Chem. Eng. 65, 922–927 (1987)
Jeyaseelan, S., Qing, L.G.: Development of adsorbent/catalyst from municipal wastewater sludge. Water Sci. Technol. 34(3–4), 499–505 (1996)
Chen, X., Jeyaseelan, S., Graham, N.: Physical and chemical properties study of the activated carbon made from sewage sludge. Waste Manag. 22, 755–760 (2002)
Martin, M.J., Artola, A., Balaguer, M.D., Rigola, M.: Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chem. Eng. J. 94(3), 231–239 (2003)
Rozada, F., Calvo, L.F., Garcia, A.I., Martin-Villacorta, J., Otero, M.: Dye adsorption by sewage sludge-based activated carbons in batch and fixed-bed systems. Bioresour Technol. 87, 221–230 (2003)
Jiang, B.Q., Xiao, Z.Q.: Preparation of copper oxide loaded activated carbon by waste sawdust for acid red GR wastewater treatment. Adv. Mater. Res. 356–360, 395–398 (2011)
Johns, M.M., Marshall, W.E., Toles, C.A.: Agricultural by-products as granular activated carbons for adsorbing dissolved metals and organics. J. Chem. Technol. Biotechnol. 71, 131–140 (1998)
Ahmedna, M., Marshall, W.E., Rao, R.M.: Granular Activated Carbons From Agricultural By-Products: Preparation, Properties and Application in Cane Sugar Refining. Louisiana State University Agricultural Center, Bulletin No. 869 (2000)
Ahmad, A.A., Hameed, B.H.: Effect of preparation conditions of activated carbon from bamboo waste for real textile wastewater. J. Hazard. Mater. 173, 487–493 (2010)
Lima, I.M., Marshall, W.E.: Production of granular activated carbons from pig manure for metal ions adsorption. J. Residuals Sci. Technol. 4, 9–16 (2007)
Wagner, N.J., Jula, R.J.: Activated carbon adsorption. In: Perrich, J.R. (ed.). Activated Carbon Adsorption for Wastewater Treatment. CRC Press, Boca Raton, Florida, pp. 41–60 (1981)
Tay, T., Ucar, S., Karagoz, S.: Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 165, 481–485 (2009)
Lima, I.M., Marshall, W.E.: Adsorption of selected environmentally important metals by poultry manure-based granular activated carbons. J. Chem. Technol. Biotechnol. 80, 1054–1061 (2005)
Qui, G., Guo, M.: Quality of poultry litter-derived granular activated carbon. Bioresour. Technol. 101, 379–386 (2010)
Fitzmorris, K.B., Lima, I.M., Marshall, W.E., Reimers, R.S.: Anion and cation leaching or desorption from activated carbons from municipal sludge and poultry manure as effected by pH. Water Environ. Res. 78, 2324–2392 (2006)
Ahmedna, M., Marshall, W.E., Husseiny, A.A., Rao, R.M., Goktepe, I.: The use of nutshell carbons in drinking water filters for removal of trace metals. Water Res. 38, 1062–1068 (2004)
Chia, C.H., Gong, B., Joseph, S.D., Marjo, C.E., Munroe, P., Rich, A.M.: Imaging of mineral-enriched biochar by FTIR, Raman and SEM-EDX. Vib. Spectrosc. 62, 248–257 (2012)
Hwang, I.H., Nakajima, D., Matsuto, T., Sugimoto, T.: Improving the quality of waste-derived char by removing ash. Waste Manage. 28, 424–434 (2008)
Bottani, E., Tascon, J. (eds.): Adsorption by Carbon, 1st edn. Elsevier, Amsterdam (2008)
Snoeyink, V.L., Jenkins, D.: Water Chemistry. Wiley, New York (1980)
Sawyer, C.N., McCarty, P.L., Parkin, G.F.: Chemistry for Environmental Engineering, 4th edn. McGraw Hill, Inc, New York (1994)
Noh, J.S., Schwarz, J.A.: Effect of HNO3 treatment of the surface of activated carbon. Carbon 28(5), 675–683 (1990)
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer: Mention of names of companies or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture over others not mentioned.
Rights and permissions
About this article
Cite this article
Brisolara, K.F., Lima, I.M. & Marshall, W.E. Cation and Anion Release from Broiler Litter and Cake Activated Carbons and the Role of Released Anions in Copper Ion Uptake. Waste Biomass Valor 5, 689–697 (2014). https://doi.org/10.1007/s12649-013-9258-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-013-9258-3