Abstract
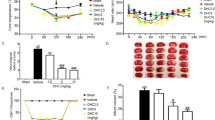
Ischemic preconditioning (IPC) could protect the blood–brain barrier (BBB), but the underlying mechanism is not well understood. This preclinical study aimed to investigate whether glycocalyx could be involved in the neuroprotective effect of IPC on cerebral ischemia–reperfusion injury (IRI) and the possible mechanism in rat middle cerebral artery occlusion/reperfusion (MCAO/R) model. Neurological deficit scores, infarct volume, and brain edema were measured to assess the neuroprotection of IPC. Several serum biomarkers related to glycocalyx damage, such as hyaluronic acid (HA), heparan sulfate (HS), and syndecan-1 (SYND1), were evaluated, and their changes were normalized to the ratio of postoperative/preoperative concentration. Western blot and immunofluorescence were used to evaluate the content and cellular location of HA-related metabolic enzymes. This study found that (1) IPC improved brain infarction and edema, neurological impairment, and BBB disruption in IRI rats; (2) IPC significantly up-regulated HA ratio and down-regulated HS ratio, but did not affect SYND1 ratio compared with the IRI group. Moreover, the increased HA ratio was negatively related to brain edema and neurological deficit score. (3) IPC affected HA metabolism by up-regulating hyaluronate synthase-1 and matrix metalloproteinase-2, and down-regulating hyaluronidase-1 in brain tissue. Together, this is the first report that the neuroprotective effect of IPC on IRI may be mediated through interfering with glycocalyx in the MCAO/R model.






Similar content being viewed by others
Data Availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
Pico F, Lapergue B, Ferrigno M, et al. Effect of in-hospital remote ischemic perconditioning on brain infarction growth and clinical outcomes in patients with acute ischemic stroke: the RESCUE BRAIN randomized clinical trial. JAMA Neurol. 2020;77:725–34. https://doi.org/10.1001/jamaneurol.2020.0326.
Przykaza Ł. Understanding the connection between common stroke comorbidities, their associated inflammation, and the course of the cerebral ischemia/reperfusion cascade. Front Immunol. 2021;12:782569. https://doi.org/10.3389/fimmu.2021.782569.
Bernardo-Castro S, Sousa J, Brás A, et al. Pathophysiology of blood-brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front Neurol. 2020;11:594672. https://doi.org/10.3389/fneur.2020.594672.
Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci USA. 2018;115:E9429–38. https://doi.org/10.1073/pnas.1802155115.
Abdullahi W, Tripathi D, Ronaldson P. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315:C343–56. https://doi.org/10.1152/ajpcell.00095.2018.
Liu S, Agalliu D, Yu C, et al. The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des. 2012;18:3653–62. https://doi.org/10.2174/138161212802002706.
Ronaldson P, Davis T. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des. 2012;18:3624–44. https://doi.org/10.2174/138161212802002625.
Schött U, Solomon C, Fries D, et al. The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med. 2016;24:48. https://doi.org/10.1186/s13049-016-0239-y.
Reitsma S, Slaaf D, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–59. https://doi.org/10.1007/s00424-007-0212-8.
Abassi Z, Armaly Z, Heyman S. Glycocalyx degradation in ischemia-reperfusion injury. Am J Pathol. 2020;190:752–67. https://doi.org/10.1016/j.ajpath.2019.08.019.
Li X, Zhu J, Liu K, et al. Heparin ameliorates cerebral edema and improves outcomes following status epilepticus by protecting endothelial glycocalyx in mice. Exp Neurol. 2020;330:113320. https://doi.org/10.1016/j.expneurol.2020.113320.
Li S, Hafeez A, Noorulla F, et al. Preconditioning in neuroprotection: from hypoxia to ischemia. Prog Neurobiol. 2017;157:79–91. https://doi.org/10.1016/j.pneurobio.2017.01.001.
Sprick J, Mallet R, Przyklenk K, et al. Ischaemic and hypoxic conditioning: potential for protection of vital organs. Exp Physiol. 2019;104:278–94. https://doi.org/10.1113/ep087122.
Petullo D, Masonic K, Lincoln C, et al. Model development and behavioral assessment of focal cerebral ischemia in rats. Life Sci. 1999;64:1099–108. https://doi.org/10.1016/s0024-3205(99)00038-7.
Zhu J, Li X, Yin J, et al. Glycocalyx degradation leads to blood-brain barrier dysfunction and brain edema after asphyxia cardiac arrest in rats. J Cereb Blood Flow Metab. 2018;38:1979–92. https://doi.org/10.1177/0271678x17726062.
Rancan L, Simón C, Sánchez Pedrosa G, et al. Glycocalyx degradation after pulmonary transplantation surgery. Eur Surg Res Eur Chir Forsch Rech Chirurg Eur. 2018;59:115–25. https://doi.org/10.1159/000489492.
Bongoni A, Lu B, McRae J, et al. Complement-mediated damage to the glycocalyx plays a role in renal ischemia-reperfusion injury in mice. Transplant Direct. 2019;5:e341. https://doi.org/10.1097/txd.0000000000000881.
Annecke T, Fischer J, Hartmann H, et al. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischaemia/reperfusion. Br J Anaesth. 2011;107:679–86. https://doi.org/10.1093/bja/aer269.
Zhu J, Li Z, Ji Z, et al. Glycocalyx is critical for blood-brain barrier integrity by suppressing caveolin1-dependent endothelial transcytosis following ischemic stroke. Brain Pathol (Zurich, Switzerland). 2022;32:e13006. https://doi.org/10.1111/bpa.13006.
Gao L, Lipowsky H. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. https://doi.org/10.1016/j.mvr.2010.06.005.
Vogel J, Sperandio M, Pries A, et al. Influence of the endothelial glycocalyx on cerebral blood flow in mice. J Cereb Blood Flow Metab. 2000;20:1571–8. https://doi.org/10.1097/00004647-200011000-00007.
Ando Y, Okada H, Takemura G, et al. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8:17523. https://doi.org/10.1038/s41598-018-35976-2.
Wang G, Tiemeier G, van den Berg B, et al. Endothelial glycocalyx hyaluronan: regulation and role in prevention of diabetic complications. Am J Pathol. 2020;190:781–90. https://doi.org/10.1016/j.ajpath.2019.07.022.
Fowke T, Karunasinghe R, Bai J, et al. Hyaluronan synthesis by developing cortical neurons in vitro. Sci Rep. 2017;7:44135. https://doi.org/10.1038/srep44135.
Lorenzl S, Albers D, Narr S, et al. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson’s disease. Exp Neurol. 2002;178:13–20. https://doi.org/10.1006/exnr.2002.8019.
Al Qteishat A, Gaffney J, Krupinski J, et al. Hyaluronan expression following middle cerebral artery occlusion in the rat. NeuroReport. 2006;17:1111–4. https://doi.org/10.1097/01.wnr.0000227986.69680.20.
Walker E, Rosenberg G. Divergent role for MMP-2 in myelin breakdown and oligodendrocyte death following transient global ischemia. J Neurosci Res. 2010;88:764–73. https://doi.org/10.1002/jnr.22257.
Xiang J, Andjelkovic A, Zhou N, et al. Is there a central role for the cerebral endothelium and the vasculature in the brain response to conditioning stimuli? Conditioning Med 2018;1:220–232.
Ju R, Wen Y, Gou R, et al. The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplant. 2014:S83–95. https://doi.org/10.3727/096368914x684998.
Sanchez-Rojas L, Gómez-Pinedo U, Benito-Martin M, et al. Biohybrids of scaffolding hyaluronic acid biomaterials plus adipose stem cells home local neural stem and endothelial cells: Implications for reconstruction of brain lesions after stroke. J Biomed Mater Res B Appl Biomater. 2019;107:1598–606. https://doi.org/10.1002/jbm.b.34252.
Stylli S, Kaye A, Novak U. Induction of CD44 expression in stab wounds of the brain: long term persistence of CD44 expression. J Clin Neurosci. 2000;7:137–40. https://doi.org/10.1054/jocn.1999.0187.
Struve J, Maher P, Li Y, et al. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia. 2005;52:16–24. https://doi.org/10.1002/glia.20215.
Fraser J, Laurent T, Laurent U. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. https://doi.org/10.1046/j.1365-2796.1997.00170.x.
Perkins K, Arranz A, Yamaguchi Y, et al. Brain extracellular space, hyaluronan, and the prevention of epileptic seizures. Rev Neurosci. 2017;28:869–92. https://doi.org/10.1515/revneuro-2017-0017.
Funding
This work was supported by grants from the National Natural Science Foundation of China (8207147) and the Science and Technology Project Plan of Liao Ning Province (2019JH2/10300027).
Author information
Authors and Affiliations
Contributions
H. S. C. designed the experiment and critically edited the manuscript. Y. N. Z., Q. W., and N. N. Z. performed the experiment. Y. N. Z. wrote the draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was approved by the Institutional Animal Care and Use Committee of General Hospital of Northern Theater Command (Ethics Number: 2019017).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, YN., Wu, Q., Zhang, NN. et al. Ischemic Preconditioning Alleviates Cerebral Ischemia–Reperfusion Injury by Interfering With Glycocalyx. Transl. Stroke Res. 14, 929–940 (2023). https://doi.org/10.1007/s12975-022-01081-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01081-w