Abstract
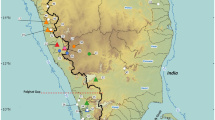
We investigated the phylogeography of the main lineages in the tadpole shrimp Triops mauritanicus Ghigi in the south-western Iberian Peninsula, using mitochondrial 12S and 16S rDNA sequences. Our results indicate that a fourth, hitherto unknown main phylogenetic lineage occurs in Iberia, so that in total, the species is divided into six distinct clades, comprising T. m. mauritanicus, T. m. simplex Ghigi, and four as yet unnamed lineages that appear to be endemic to Iberia. Percentages of sequence divergence among the main clades in T. mauritanicus reach the range reported for recognized species in other notostracan lineages. A thorough morphological investigation also revealed that the differentiation among these lineages is higher than previously thought, and that populations of three of the main clades within T. mauritanicus can be reliably separated from each other and from the remaining lineages based on the morphology of adult males. The remaining clades also show a significant level of morphological differentiation, but include a certain proportion of populations for which the additional application of molecular methods is needed for a reliable determination. The geographic distributions of 12S haplotypes are indicative of frequent dispersal events and gene flow among populations belonging to the same main lineage, but give no evidence of recent migration events among different main lineages, suggesting that there is no gene flow among the latter. Our data thus suggest that the six main lineages within T. mauritanicus represent distinct species. We therefore describe the Iberian lineages as T. baeticus Korn n. sp., T. emeritensis Korn & Pérez-Bote n. sp., T. gadensis Korn & García-de-Lomas n. sp., and T. vicentinus Korn, Machado, Cristo & Cancela da Fonseca n. sp., and reinstate T. simplex Ghigi to full species status. Our data confirm the general, previously recognized pattern of a lower dispersal probability in gonochoric Triops taxa. However, we found evidence that passive dispersal in Triops may be further complicated by a strong habitat dependence of dispersal probability, mediated by prevailing dispersal vectors.










Similar content being viewed by others
References
Alonso, M. (1985). A survey of the Spanish euphyllopoda. Miscel·lània Zoològica, 9, 179–208.
Ballard, J. W., Olsen, G. J., Faith, D. P., Odgers, W. A., Rowell, D. M., & Atkinson, P. W. (1992). Evidence from 12S ribosomal RNA sequences that onychophorans are modified arthropods. Science, 258, 1345–1348.
Barnard, K. H. (1929). A revision of the South African branchiopoda phyllopoda. Annals. South African Museum, 29, 181–272.
Bohonak, A. J., & Jenkins, D. G. (2003). Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecological Letters, 6, 783–796.
Cancela da Fonseca, L., Cristo, M., Machado, M., Sala, J., Reis, J., Alcazar, R., et al. (2008). Mediterranean temporary ponds in southern Portugal: key faunal groups as management tools? Pan-American Journal of Aquatic Sciences, 3, 304–320.
Colbourne, J. K., Wilson, C. C., & Hebert, P. D. N. (2006). The systematics of Australian Daphnia and Daphniopsis (Crustacea: Cladocera): a shared phylogenetic history transformed by habitat-specific rates of evolution. Biological Journal of the Linnean Society, 89, 469–488.
De Meester, L., Gómez, A., Okamura, B., & Schwenk, K. (2002). The monopolization hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecologica, 23, 121–135.
Eriksson, J., Hohmann, G., Boesch, C., & Vigilant, L. (2004). Rivers influence the population genetic structure of bonobos (Pan paniscus). Molecular Ecology, 13, 3425–3435.
Fletcher, W. J., Boski, T., & Moura, D. (2007). Palynological evidence for environmental and climatic change in the lower Guadiana valley, Portugal, during the last 13 000 years. Holocene, 17, 481–494.
Fletcher, W. J., & Sánchez Goñi, M. F. (2008). Orbital- and sub-orbital-scale climate impacts on vegetation of the western Mediterranean basin over the last 48,000 yr. Quaternary Research, 70, 451–464.
Fortelius, M. [coord.] (2003). Neogene of the Old World database of fossil mammals (NOW). University of Helsinki. http://www.helsinki.fi/science/now/. Accessed 10 September 2009.
Frantz, A. C., Pope, L. C., Etherington, T. R., Wilson, G. J., & Burke, T. (2010). Using isolation-by-distance-based approaches to assess the barrier effect of linear landscape elements on badger (Meles meles) dispersal. Molecular Ecology, 19, 1663–1674.
Frisch, D., Green, A. J., & Figuerola, J. (2007). High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquatic Science, 69, 568–574.
Fryer, G. (1988). Studies on the functional morphology and biology of the Notostraca (Crustacea: Branchiopoda). Philosophical Transactions of the Royal Society of London, Series B, 321, 27–124.
Ghigi, A. (1921). Ricerche sui Notostraci di Cirenaica e di altri paesi del Mediterraneo. Atti della Società Italiana di Scienze Naturali, 60, 161–188.
Granados Corona, M., Martin Vicente, A., & García Novo, F. (1988). Long-term vegetation changes on the stabilized dunes of Doñana National Park (SW Spain). Vegetatio, 75, 73–80.
Green, A. J., & Figuerola, J. (2005). Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Diversity and Distributions, 11, 149–156.
Green, A. J., Jenkins, K. M., Bell, D., Morris, P. J., & Kingsford, R. T. (2008). The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshwater Biology, 53, 380–392.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Hamer, M., & Rayner, N. A. (1995). A note on the taxonomy and distribution of Triops Schrank (Crustacea: Branchiopoda: Notostraca) in southern Africa. Annals of the Natal Museum, 36, 9–19.
Hartnoll, R. G. (1978). The determination of relative growth in Crustacea. Crustaceana, 34, 281–293.
Hawes, T. C. (2009). Origins and dispersal of the Antarctic fairy shrimp. Antarctic Science, 21, 477–482.
Huelsenbeck, J. P., & Ronquist, F. (2001). MRBAYES: bayesian inference of phylogeny. Bioinformatics, 17, 754–755.
Ishida, S., & Taylor, D. J. (2007). Mature habitats associated with genetic divergence despite strong dispersal ability in an arthropod. BMC Evolutionary Biology, 7, 52.
Kazantzidis, S., & Goutner, V. (2005). The diet of nestlings of three Ardeidae species (Aves, Ciconiiformes) in the Axios Delta, Greece. Belgian Journal of Zoology, 135, 165–170.
Korn, M., & Hundsdoerfer, A. K. (2006). Evidence for cryptic species in the tadpole shrimp Triops granarius (Lucas, 1864) (Crustacea: Notostraca). Zootaxa, 1257, 57–68.
Korn, M., Marrone, F., Pérez-Bote, J. L., Machado, M., Cristo, M., Cancela da Fonseca, L., et al. (2006). Sister species within the Triops cancriformis lineage (Crustacea, Notostraca). Zoologica Scripta, 35, 301–322.
Krapu, G. L., & Swanson, G. A. (1977). Foods of juvenile, brood hen, and post-breeding pintails in North Dakota. The Condor, 79, 504–507.
Kumar, S., Tamura, K., Jakobsen, I. B., & Nei, M. (2001). MEGA2—molecular evolutionary genetics analysis. Tempe: Arizona State University.
Kurtén, B. (1968). Pleistocene mammals of Europe. [Reprint 2008]. New Brunswick: Aldine Transaction.
Lance, R. F., Kennedy, M. L., & Leberg, P. (2000). Classification bias in discriminant function analyses used to evaluate putatively different taxa. Journal of Mammalogy, 81, 245–249.
Linder, F. (1952). Contributions to the morphology and taxonomy of the Branchiopoda Notostraca, with special reference to the North American species. Proceedings of the United States National Museum, 102, 1–69.
Lo, P. L. (1991). Diet of the White-faced Heron on Manawatu pastures. Notornis, 38, 63–71.
Longhurst, A. R. (1955). A review of the Notostraca. Bulletin of the British Museum (Natural History). Zoology, 3, 1–57.
Lynch, J. E. (1972). Lepidurus couesii Packard (Notostraca) redescribed with a discussion of specific characters in the genus. Crustaceana, 23, 43–49.
Mantovani, B., Cesari, M., & Scanabissi, F. (2004). Molecular taxonomy and phylogeny of the ´living fossil´ lineages Triops and Lepidurus (Branchiopoda: Notostraca). Zoologica Scripta, 33, 367–374.
Mokany, A., Wood, J. T., & Cunningham, S. A. (2008). Effect of shade and shading history on species abundances and ecosystem processes in temporary ponds. Freshwater Biology, 53, 1917–1928.
Montes, C., Borja, F., Bravo, M. A., & Moreira, J. M. [Coords.]. (1998). Reconocimiento biofísico de espacios naturales protegidos. Doñana: una aproximación ecosistémica. Sevilla: Junta de Andalucía.
Moorhead, D. L., Hall, D. L., & Willig, M. R. (1998). Succession of macroinvertebrates in playas of the Southern High Plains, USA. Journal of the North American Benthological Society, 17, 430–442.
Nei, M. (1987). Molecular evolutionary genetics. New York: Columbia University Press.
Pérez-Bote, J. L., Muñoz, A., García, J. M., Rodríguez, S. P., Romero, A. J., Corbacho, P., et al. (2006). Distribución, estatus y conservación de los grandes branchiópodos (Crustacea, Branchiopoda) en Extremadura (SO de la Península Ibérica). Boletín de la Asociación Española de Entomología, 30, 41–57.
Pérez-Hurtado, A., Hortas, F., Ruiz, J., & Solís, F. (1993). Importancia de la Bahía de Cádiz para las poblaciones de limícolas invernantes e influencia de las transformaciones humanas. Ardeola, 40, 133–142.
Posada, D., & Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics, 14, 817–818.
Proctor, V. W. (1964). Viability of crustacean eggs recovered from ducks. Ecology, 45, 656–658.
Quinn, G. P., & Keough, M. J. (2003). Experimental design and data analysis for biologists. Reprint with corrections. Cambridge: Cambridge University Press.
Raes, J., & Van de Peer, Y. (1998). ForCon 1.0 for windows. Antwerp: University of Antwerp.
Rendón, M. A., Green, A. J., Aguilera, E., & Almaraz, P. (2008). Status, distribution and long-term changes in the waterbird community wintering in Doñana, south-west Spain. Biological Conservation, 141, 1371–1388.
Rogers, D. C. (2001). Revision of the Nearctic Lepidurus (Notostraca). Journal of Crustacean Biology, 21, 991–1006.
Sánchez, M. I., Green, A. J., Amat, F., & Castellanos, E. M. (2007). Transport of brine shrimps via the digestive system of migratory waders: dispersal probabilities depend on diet and season. Marine Biology, 151, 1407–1415.
Sassaman, C., Simovich, M. A., & Fugate, M. (1997). Reproductive isolation and genetic differentiation in North American species of Triops (Crustacea: Branchiopoda: Notostraca). Hydrobiologia, 359, 125–147.
Schneider, S., Roessli, D., & Excoffier, L. (2000). Arlequin, version 2.000. A software for population genetics data analysis. Geneva: University of Geneva, Genetics & Biometry Laboratory.
Schram, F. R., & Koenemann, S. (2004). Developmental genetics and arthropod evolution: On body regions of Crustacea. In G. Scholtz (Ed.), Evolutionary developmental biology of Crustacea, Crustacean Issues, 15 (pp. 75–92). Lisse: Balkema.
Serrano, L., Reina, M., Martín, G., Reyes, I., Arechederra, A., León, D., et al. (2006). The aquatic systems of Doñana (SW Spain): watersheds and frontiers. Limnetica, 25, 11–32.
Stamatakis, A., Hoover, P., & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web-servers. Systems Biology, 75, 758–771.
Suno-Uchi, N., Sasaki, F., Chiba, S., & Kawata, M. (1997). Morphological stasis and phylogenetic relationships in tadpole shrimps, Triops (Crustacea: Notostraca). Biological Journal of the Linnean Society, 61, 439–457.
Swofford, D. L. (2003). PAUP*—phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland: Sinauer.
Thiéry, A. (1987). Les crustacés branchiopodes Anostraca Notostraca & Conchostraca des milieux limniques temporaires (dayas) au Maroc. Taxonomie, biogéographie, écologie. Doctoral thesis. Marseille: Université Aix-Marseille III.
Thompson, J. D. (2005). Plant evolution in the Mediterranean. New York: Oxford University Press.
Tourenq, C., Aulagnier, S., Durieux, L., Lek, S., Mesléard, F., Johnson, A., et al. (2001). Identifying rice fields at risk from damage by the Greater Flamingo. Journal of Applied Ecology, 38, 170–179.
Vanschoenwinkel, B., Waterkeyn, A., Vandecaetsbeek, T., Pineau, O., Grillas, P., & Brendonck, L. (2008). Dispersal of freshwater invertebrates by large terrestrial mammals: a case study with wild boar (Sus scrofa) in Mediterranean wetlands. Freshwater Biology, 53, 2264–2273.
Vekhoff, N. V. (1993). The fauna and zoogeography of fairy and tadpole shrimps of Russia and adjacent lands (Crustacea Anostraca, Notostraca). Arthropoda Selecta, 2, 11–42.
Xia, X., & Xie, Z. (2001). DAMBE: data analysis in molecular biology and evolution. The Journal of Heredity, 92, 371–373.
Zierold, T., Hanfling, B., & Gómez, A. (2007). Recent evolution of alternative reproductive modes in the ‘living fossil’ Triops cancriformis. BMC Evolutionary Biology, 7, 161.
Zwickl, D. J. (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the Maximum Likelihood criterion. PhD dissertation. Austin: University of Texas. [Genetic algorithm for rapid likelihood inference software available from http://www.bio.utexas.edu/faculty/antisense/garli/Garli.html].
Acknowledgements
We are very grateful to Miguel Alonso (Barcelona), Federico Marrone (Palermo), Yasar Al-Khalili (Pest Management Consultants, Dubai) and Ernst-Gerhard Burmeister (München) for providing samples for this investigation, to Hugues Lefranc (Sevilla) for his help in taking samples, and to David Paz and Hector Garrido (Sevilla) for assistance in the field. We are greatly indebted to Christian Kehlmaier and Anke Müller (Dresden) for their dedicated engagement in the sequencing work. Katja Frohberg (Dresden) further contributed to the sequencing work in this study. We are grateful to two anonymous reviewers, as well as to Olaf R.P. Bininda-Emonds (Chief Editor) and Martin V. Sørensen (Associate Editor) for their most helpful suggestions that greatly improved the manuscript. We would further like to thank Ian J. Kitching (London) for advice on the taxonomy section.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
(XLS 128 kb)
Appendix
Appendix
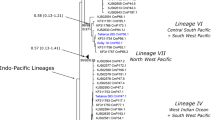
ML tree based on COI sequences (RAxML program, setting ‘estimate proportion of invariable sites’; best evolutionary model obtained by Modeltest was TrN+I+G, selected by AIC). ML bootstrap support (obtained with RAxML) given for selected branches. Outgroups [GenBank sequences of Lepidurus apus (accession number EF189669), L. arcticus (AF209067), L. couesii (DQ310622), L. lemmoni (GQ144447), Triops longicaudatus (DQ310623 and GQ144444), T. australiensis (DQ889135), T. granarius (GQ144446)] removed for clarity. Samples labelled, as applicable, with short names of main phylogenetic lineages (Table A1) followed by museum specimen tissue voucher numbers (MTD-TW; sequences submitted to GenBank, acc. nrs. FN691430–FN691444) or by GenBank accessions, or labelled with GenBank accessions containing numbers but no lineage data (samples with GenBank taxon labels apparently resulting from erroneous species identification, i.e. samples submitted to GenBank with invalid species names). Abbreviations: T.c. = Triops cancriformis; T.m. = T. mauritanicus
Rights and permissions
About this article
Cite this article
Korn, M., Green, A.J., Machado, M. et al. Phylogeny, molecular ecology and taxonomy of southern Iberian lineages of Triops mauritanicus (Crustacea: Notostraca). Org Divers Evol 10, 409–440 (2010). https://doi.org/10.1007/s13127-010-0026-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-010-0026-y