Abstract
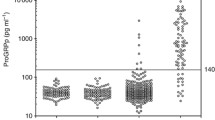
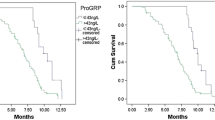
Progastrin-releasing peptide (proGRP) is a promising serum tumor marker for small cell lung cancer (SCLC). We have tested assay specificity and performed a correlation study between a recently developed time-resolved immunofluorometric assay (TR-IFMA) for proGRP and the established Advanced Life Science Institute (ALSI) ELISA method. Between-method correlation and comparison of clinical performance were studied in 481 individuals, among them, 178 lung cancers, 84 benign diseases of the lung, and 219 healthy controls. Follow-up time >6 years was observed for 89 patients with SCLC. The two assays had quite different epitope specificities where the TR-IFMA recognized a considerable smaller proGRP fragment than the ALSI ELISA. However, the correlation between the two methods for elevated proGRP values (>85 ng/l) was good (ρ = 0.948). Both assays displayed good discrimination between benign lung diseases and SCLC. The cut-off values for positive classification of SCLC versus non-small cell lung cancers and benign lung diseases at >95% specificity were 85 ng/l for the TR-IFMA and 42 ng/l for the ALSI ELISA. Both proGRP assays showed good clinical validity. However, due to differences in the recommended cut-off values, switching methods is not recommended. There was a significant difference in survival of patients with TR-IFMA proGRP values over the cut-off (85 ng/l) compared with patients with values under the cut-off, p = 0.0002. In contrast, the ALSI ELISA assay failed to provide statistically significant prognostic information, p = 0.066.





Similar content being viewed by others
References
Jorgensen LG, Osterlind K, Hansen HH, Cooper EH. Serum neuron-specific enolase (S-NSE) in progressive small-cell lung cancer (SCLC). Br J Cancer. 1994;70:759–61.
Jorgensen LG, Osterlind K, Genolla J, Gomm SA, Hernandez JR, Johnson PW, et al. Serum neuron-specific enolase (S-NSE) and the prognosis in small-cell lung cancer (SCLC): a combined multivariable analysis on data from nine centres. Br J Cancer. 1996;74:463–7.
Molina R, Auge JM, Filella X, Vinolas N, Alicarte J, Domingo JM, et al. Pro-gastrin-releasing peptide (proGRP) in patients with benign and malignant diseases: comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res. 2005;25:1773–8.
Niho S, Nishiwaki Y, Goto K, Ohmatsu H, Matsumoto T, Hojo F, et al. Significance of serum pro-gastrin-releasing peptide as a predictor of relapse of small cell lung cancer: comparative evaluation with neuron-specific enolase and carcinoembryonic antigen. Lung Cancer. 2000;27:159–67.
Stieber P, Dienemann H, Schalhorn A, Schmitt UM, Reinmiedl J, Hofmann K, et al. Pro-gastrin-releasing peptide (ProGRP)—a useful marker in small cell lung carcinomas. Anticancer Res. 1999;19:2673–8.
McDonald TJ, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. A gastrin releasing peptide from the porcine nonantral gastric tissue. Gut. 1978;19:767–74.
Spindel ER, Chin WW, Price J, Rees LH, Besser GM, Habener JF. Cloning and characterization of cDNAs encoding human gastrin-releasing peptide. Proc Natl Acad Sci U S A. 1984;81:5699–703.
Sausville EA, Lebacq-Verheyden AM, Spindel ER, Cuttitta F, Gazdar AF, Battey JF. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J Biol Chem. 1986;261:2451–7.
Reeve Jr JR, Cuttitta F, Vigna SR, Heubner V, Lee TD, Shively JE, et al. Multiple gastrin-releasing peptide gene-associated peptides are produced by a human small cell lung cancer line. J Biol Chem. 1989;264:1928–32.
Patel O, Dumesny C, Shulkes A, Baldwin GS. C-terminal fragments of the gastrin-releasing peptide precursor stimulate cell proliferation via a novel receptor. Endocrinology. 2007;148:1330–9.
Nordlund MS, Warren DJ, Laerdahl JK, Paus E. Studies on multiple forms of proGRP in serum from small cell lung cancer patients. Tumour Biol. 2009;30:265–75.
Nordlund MS, Fermer C, Nilsson O, Warren DJ, Paus E. Production and characterization of monoclonal antibodies for immunoassay of the lung cancer marker proGRP. Tumour Biol. 2007;28:100–10.
Aoyagi K, Miyake Y, Urakami K, Kashiwakuma T, Hasegawa A, Kodama T, et al. Enzyme immunoassay of immunoreactive progastrin-releasing peptide(31-98) as tumor marker for small-cell lung carcinoma: development and evaluation. Clin Chem. 1995;41:537–43.
Nordlund MS, Warren DJ, Nustad K, Bjerner J, Paus E. Automated time-resolved immunofluorometric assay for progastrin-releasing peptide. Clin Chem. 2008;54:919–22.
Nordlund MS, Bjerner J, Warren DJ, Nustad K, Paus E. Progastrin-releasing peptide: stability in plasma/serum and upper reference limit. Tumour Biol. 2008;29:204–10.
Warren DJ, Nordlund MS, Paus E. Formulation of immunoassay calibrators in pasteurized albumin can significantly enhance their durability. J Immunol Methods. 2009;353:145–7.
Yoshimura T, Fujita K, Kinukawa H, Matsuoka Y, Patil RD, Beligere GS, et al. Development and analytical performance evaluation of an automated chemiluminescent immunoassay for pro-gastrin releasing peptide (ProGRP). Clin Chem Lab Med. 2009;47:1557–63.
Reeve Jr JR, Cuttitta F, Vigna SR, Shively JE, Walsh JH. Processing of mammalian preprogastrin-releasing peptide. Ann N Y Acad Sci. 1988;547:21–9.
Lebacq-Verheyden AM, Kasprzyk PG, Raum MG, Van Wyke CK, Lebacq JA, Battey JF. Posttranslational processing of endogenous and of baculovirus-expressed human gastrin-releasing peptide precursor. Mol Cell Biol. 1988;8:3129–35.
Yoshimura T, Fujita K, Kawakami S, Takeda K, Chan S, Beligere G, et al. Stability of pro-gastrin-releasing peptide in serum versus plasma. Tumour Biol. 2008;29:224–30.
Bolstad N, Warren DJ, Bjerner J, Kravdal G, Schwettmann L, Olsen KH, Rustad P, Nustad K. Heterophilic antibody interference in commercial immunoassays; a screening study using paired native and pre-blocked sera. Clin Chem Lab Med 2011 (in press)
Bjerner J, Nustad K, Norum LF, Olsen KH, Bormer OP. Immunometric assay interference: incidence and prevention. Clin Chem. 2002;48:613–21.
Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81. viii.
Nisman B, Heching N, Biran H, Barak V, Peretz T. The prognostic significance of circulating neuroendocrine markers chromogranin a, pro-gastrin-releasing peptide and neuron-specific enolase in patients with advanced non-small-cell lung cancer. Tumour Biol. 2006;27:8–16.
Acknowledgments
This work was supported by the Norwegian Cancer Society. We will thank Lars Mørkrid, MD, PhD, for skilled statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nordlund, M.S., Stieber, P., Brustugun, O.T. et al. Characteristics and clinical validity of two immunoassays for ProGRP. Tumor Biol. 33, 1105–1113 (2012). https://doi.org/10.1007/s13277-012-0351-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0351-1