Abstract
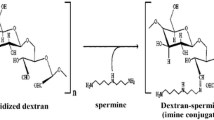
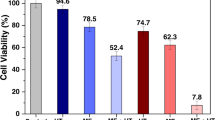
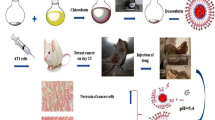
Dextran-functionalized maghemite fluid (DexMF) has been tested to treat Ehrlich-solid-tumor-bearing mice, evidencing its potential use in mediating magnetohyperthermia in breast cancer treatment. However, although magnetic nanoparticles tend to accumulate in tumor tissues, part of the nanomaterial can reach the blood stream, and then the organism. The aim of this study was to investigate the acute systemic effects of the intratumoral injection of DexMF mediating magnetohyperthermia in the treatment of an advanced clinical Ehrlich-solid-tumor, assessed through histopathological analyses of liver, kidneys, heart and spleen, comet assay, micronucleus test, hemogram, and serum levels of bilirubin, aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase, alkaline phosphatase, creatinine, and urea. The tumor’s histopathology and morphometry were used to assess its aggressiveness and regression. DexMF mediating hyperthermia was effective in containing tumor aggressiveness and in inducing tumor regression, besides showing no toxic effects. Its physical characteristics also suggest that it is safe to use in other biomedical applications.






Similar content being viewed by others
References
World Health Organization (WHO). Breast Cancer Awareness Month in October. http://www.who.int/cancer/events/breast_cancer_month/en/. Accessed April 22, 2013 2013.
Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6(12):718–30.
Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchió C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. 2010;57(2):171–92.
Dhankhar R, Vyas SP, Jain AK, Arora S, Rath G, Goyal AK. Advances in Novel Drug Delivery Strategies for Breast Cancer Therapy. Artif Cells Blood Substit Immobil Biotechnol. 2010;38(5):230–49. doi:10.3109/10731199.2010.494578.
Verrill M. Chemotherapy for early-stage breast cancer: a brief history. Br J Cancer. 2009;101(S1):S2–5.
Rastogi A. Nanooncology: The Breast Cancer Story. UTMJ. 2011;88(3):190–4.
Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–40. doi:10.1016/s1470-2045(00)00254-0.
Carneiro MLB, Peixoto R, Joanitti G, Oliveira R, Telles L, Miranda-Vilela AL, et al. Antitumor effect and toxicity of free rhodium (II) citrate and rhodium (II) citrate-loaded maghemite nanoparticles in mice bearing breast cancer. J Nanobiotechnol. 2013;11(1):4.
Miranda-Vilela AL, Peixoto RCA, Longo JPF, e Cintra DOS, Portilho FA, Miranda KLC, et al. Dextran-Functionalized Magnetic Fluid Mediating Magnetohyperthermia Combined with Preventive Antioxidant Pequi-Oil Supplementation: Potential Use Against Cancer. J Biomed Nanotechnol. 2013;9(7):1261–71. doi:10.1166/jbn.2013.1616.
Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003;36:R198–206.
Tartaj P, Morales MP, Veintemillas-Verdaguer S, González-Carreño T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003;36(13):R182.
Ren C. Nanotechnology and the New Age of Cancer Treatment. The National High School Journal of Science. 2012. http://nhsjs.com/2012/nanotechnology-and-the-new-age-of-cancer-treatment. Accessed April 22 2013.
O’Grady K. Biomedical applications of magnetic nanoparticles. J 703 Phys D: Appl Phys. 2002;36(13). doi:10.1088/0022-3727/36/13/701.
Silva AC, Oliveira TR, Mamani JB, Malheiros SM, Malavolta L, Pavon LF, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomedicine. 2011;6:591–603.
Portilho FA, Estevanato LLC, Miranda-Vilela AL, Almeida-Santos MFM, de Oliveira-Cavalcanti CE, Lacava BM, et al. Investigation of a magnetohyperthermia system efficacy. J Appl Phys. 2011;109:07B307.
Sarfati G, Dvir T, Elkabets M, Apte RN, Cohen S. Targeting of polymeric nanoparticles to lung metastases by surface-attachment of YIGSR peptide from laminin. Biomaterials. 2011;32(1):152–61.
Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2007;15(1):3–12.
Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2(3):22–32. doi:10.1016/S1748-0132(07)70084-1.
Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39.
Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn. 1981;17(2):1247–8. doi:10.1109/tmag.1981.1061188.
Miranda-Vilela AL, Portilho FA, de Araujo VG, Estevanato LL, Mezzomo BP, Almeida Santos MF, et al. The protective effects of nutritional antioxidant therapy on Ehrlich solid tumor-bearing mice depend on the type of antioxidant therapy chosen: histology, genotoxicity and hematology evaluations. J Nutr Biochem. 2011;22(11):1091–8. doi:10.1016/j.jnutbio.2010.09.009.
American Veterinary Medical Association (AVMA). AVMA Guidelines on Euthanasia. 2007. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. Accessed August 11, 2012.
Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: an in vivo study. Jpn J Cancer Res. 1998;89(4):463–9.
Singh N, McCoy M, Tice R, Scheneider E. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91.
Estevanato L, Cintra D, Baldini N, Portilho F, Barbosa L, Martins O, et al. Preliminary biocompatibility investigation of magnetic albumin nanosphere designed as a potential versatile drug delivery system. Int J Nanomedicine. 2011;6:1709–17. doi:10.2147/IJN.S21323.
Estevanato LL, Lacava LM, Carvalho LC, Azevedo RB, Silva O, Pelegrini F, et al. Long-term biodistribution and biocompatibility investigation of dextran-coated magnetite nanoparticle using mice as the animal model. J Biomed Nanotechnol. 2011;8(2):301–8.
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63(1–2):24–46. doi:10.1016/j.addr.2010.05.006.
Berry CC. Progress in functionalization of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2009;42(22):224003. 9pp.
Nichols JW, Bae YH. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today. 2012;7(6):606–18. doi:10.1016/j.nantod.2012.10.010.
Cho W-S, Thielbeer F, Duffin R, Johansson EMV, Megson IL, MacNee W et al. Surface functionalization affects the zeta potential, coronal stability and membranolytic activity of polymeric nanoparticles. Nanotoxicology. 2013; Ahead of Print:1–10. doi:10.3109/17435390.2013.773465.
Patil S, Sandberg A, Heckert E, Self W, Seal S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 2007;28(31):4600–7. doi:10.1016/j.biomaterials.2007.07.029.
Souris JS, Lee C-H, Cheng S-H, Chen C-T, Yang C-S, Ho J-aA, et al. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials. 2010;31(21):5564–74. doi:10.1016/j.biomaterials.2010.03.048.
Stewart HL, Snell KC, Dunham LJ, Schylen SM. Transplantable and Transmissible Tumors of Animals: Atlas of Tumor Pathology. Washington: Armed Fource Institute of Pathology; 1959.
Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1(1):22–30.
Everds NE. Hematology of the Laboratory Mouse. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. San Diego: Elsevier; 2007. p. 133–70.
Mitruka BM, Rawnsley HM. Clinical, Biochemical and Haematological Reference Values in Normal Experimental Animals. New York: Masson Pub; 1977.
Viana FAB. Guia terapêutico veterinário. 2nd ed. Gráfica Editora Cem: Minas Gerais; 2007.
Organisation for Economic Co-operation and Development (OECD). OECD guideline for the testing of chemicals: Mammalian erythrocyte micronucleus test. 2012. http://www.oecd.org/env/ehs/testing/Draft%20TG%20474.pdf. Accessed May 04, 2013 2013.
Quimby FW, Luong RH. Clinical Chemistry of the Laboratory Mouse. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. San Diego: Elsevier; 2007. p. 171–216.
Sutherland RJ. Biochemical evaluation of the hepatobiliary system in dogs and cats. Vet Clin N Am Small Anim Pract. 1989;19(5):899–927.
Thrall MA, Baker DC, Campbell TW, DeNicola D, Fettman MJ, Lassen ED, et al. Hematologia e Bioquímica Clínica Veterinária. 1st Edition ed. São Paulo: Editora Roca; 2007.
Miras-Parra FJ, Parra-Ruiz J, Gómez-Morales M, Gómez-Jiménez FJ, de la Higuera-Torres-Puchol J. Inflammatory Pseudotumor of Lymph Nodes with Focal Infiltration in Liver and Spleen. Dig Dis Sci. 2003;48(10):2003–4.
Goldberg DM. Structural, functional, and clinical aspects of gamma-glutamyltransferase. CRC Crit Rev Clin Lab Sci. 1980;12(1):1–58.
Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263–355.
Acknowledgments
We are grateful to Sabin Institute/Sabin Laboratories for technical support in the biochemical dosages and the Brazilian National Council for Technological and Scientific Development (CNPq), the Foundation to Support Research in the Federal District (FAPDF), the Coordination for Further Training of Graduate Staff (CAPES), the CAPES-Rede CON-NANO, NCT-Nanobiotecnologia, and CNANO-UnB for financial support.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Miranda-Vilela, A.L., Yamamoto, K.R., Miranda, K.L.C. et al. Dextran-functionalized magnetic fluid mediating magnetohyperthermia for treatment of Ehrlich-solid-tumor-bearing mice: toxicological and histopathological evaluations. Tumor Biol. 35, 3391–3403 (2014). https://doi.org/10.1007/s13277-013-1447-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1447-y