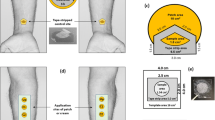
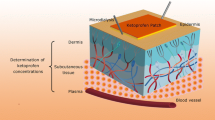
Abstract
The tissue distribution and percutaneous drug absorption of indomethacin (IND) patches were studied using commercial IND as a comparison. The concentration of IND in skin, plasma, and muscle in mice was measured by LC-MS/MS, and the IND concentration in the dermis of rats was also monitored by microdialysis. After percutaneous administration, the “double-peak” phenomenon occurred in different tissues, and the IND concentration was ranked as skin first, followed by plasma and then muscle. In particular, skin acted as a reservoir for drug release, and the “secondary hump” in tissue distribution was attributed to the subsequent release of lipophilic IND in skin. It was concluded that examination of the tissue distribution and application of a microdialysis technique provided an effective means of evaluating indomethacin pharmacokinetics.



Similar content being viewed by others
References
Sagar V, Ahamed RN. Gastric mucosal cellular changes induced by indomethacin (NSAID) in male albino rats. Indian J Exp Biol. 1999;37(4):365–9.
Xie G, Zhou D, Cheng K-W, Wong CC, Rigas B. Comparative in vitro metabolism of phospho-tyrosol-indomethacin by mice, rats and humans. Biochem Pharmacol. 2013;85(8):1195–202. doi:10.1016/j.bcp.2013.01.031.
Arroyo-Lira AG, Rodríguez-Ramos F, Chávez-Piña AE. Synergistic antinociceptive effect and gastric safety of the combination of docosahexaenoic acid and indomethacin in rats. Pharmacol Biochem Behav. 2014;122:74–81. doi:10.1016/j.pbb.2014.03.015.
Abioye AO, Issah S, Kola-Mustapha AT. Ex vivo skin permeation and retention studies on chitosan-ibuprofen-gellan ternary nanogel prepared by in situ ionic gelation technique-a tool for controlled transdermal delivery of ibuprofen. Int J Pharm. 2015;490(1–2):112–30. doi:10.1016/j.ijpharm.2015.05.030.
Tan HS, Pfister WR. Pressure-sensitive adhesives for transdermal drug delivery systems. Pharm Sci Technol Today. 1999;2(2):60–9. doi:10.1016/S1461-5347(99)00119-4.
Lee KE, Choi YJ, Oh BR, Chun IK, Gwak HS. Formulation and in vitro/in vivo evaluation of levodopa transdermal delivery systems. Int J Pharm. 2013;456(2):432–6. doi:10.1016/j.ijpharm.2013.08.044.
Morrow JD, Hill KE, Burk RF, Nammour TM, Bade KF, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–7.
Olugemo K, Solorio D, Sheridan C, Young CL. Pharmacokinetics and safety of low dose submicron indomethacin 20 and 40 mg compared with indomethacin 50 mg. Postgrad Med. 2015:223–31. doi:10.1080/00325481.2015.1000231.
De Castro WV, Marchand S, Lamarche I, Couet W. Effect of experimentally induced hypovolemia on ertapenem tissue distribution using microdialysis in rats. Eur J Pharm Sci. 2014;51:45–50. doi:10.1016/j.ejps.2013.08.017.
Konings I, Engels FK, Sleijfer S, Verweij J, Wiemer EAC, Loos WJ. Application of prolonged microdialysis sampling in carboplatin-treated cancer patients. Cancer Chemother Pharmacol. 2009;64(3):509–16. doi:10.1007/s00280-008-0898-0.
Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14(2):101–14. doi:10.1016/s0928-0987(01)00167-1.
Potts R, Guy R. Predicting skin permeability. Pharm Res. 1992;9(5):663–9. doi:10.1023/A:1015810312465.
Murphy NP. Dynamic measurement of extracellular opioid activity: status quo, challenges, and significance in rewarded behaviors. ACS Chem Neurosci. 2015;6(1):94–107. doi:10.1021/cn500295q.
Plock N, Kloft C. Microdialysis—theoretical background and recent implementation in applied life sciences. Eur J Pharm Sci. 2005;25(1):1–24. doi:10.1016/j.ejps.2005.01.017.
Tettey-Amlalo RNO, Kanfer I, Skinner MF, Benfeldt E, Verbeeck RK. Application of dermal microdialysis for the evaluation of bioequivalence of a ketoprofen topical gel. Eur J Pharm Sci. 2009;36(2–3):219–25. doi:10.1016/j.ejps.2008.09.002.
Brill MJE, Houwink API, Schmidt S, Van Dongen EPA, Hazebroek EJ, van Ramshorst B, et al. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother. 2014;69(3):715–23. doi:10.1093/jac/dkt444.
Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46(2):105–19.
Schnetz E, Fartasch M. Microdialysis for the evaluation of penetration through the human skin barrier—a promising tool for future research? Eur J Pharm Sci. 2001;12(3):165–74. doi:10.1016/s0928-0987(00)00155-x.
Chaurasia CS. In vivo microdialysis sampling: theory and applications. Biomed Chromatogr. 1999;13(5):317–32. doi:10.1002/(sici)1099-0801(199908)13:5<317::aid-bmc891>3.0.co;2-i.
Couto A, Fernandes R, Cordeiro MNS, Reis SS, Ribeiro RT, Pessoa AM. Dermic diffusion and stratum corneum: a state of the art review of mathematical models. J Control Release. 2014;177:74–83. doi:10.1016/j.jconrel.2013.12.005.
Chen J, Wang XM, Wang J, Liu GL, Tang X. Evaluation of brain-targeting for the nasal delivery of ergoloid mesylate by the microdialysis method in rats. Eur J Pharm Biopharm. 2008;68(3):694–700. doi:10.1016/j.ejpb.2007.08.013.
Azeredo FJ, Dalla Costa T, Derendorf H. Role of microdialysis in pharmacokinetics and pharmacodynamics: current status and future directions. Clin Pharmacokinet. 2014;53(3):205–12. doi:10.1007/s40262-014-0131-8.
Sinnollareddy MG, Roberts MS, Lipman J, Peake SL, Roberts JA. Influence of sustained low-efficiency diafiltration (SLED-f) on interstitial fluid concentrations of fluconazole in a critically ill patient: use of microdialysis. Int J Antimicrob Agents. 2015;46(1):121–4. doi:10.1016/j.ijantimicag.2015.02.017.
Kreilgaard M. Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev. 2002;54:S99–S121. doi:10.1016/s0169-409x(02)00117-5.
Ding P, Xu H, Wei G, Zheng J. Microdialysis sampling coupled to HPLC for transdermal delivery study of ondansetron hydrochloride in rats. Biomed Chromatogr. 2000;14(3):141–3. doi:10.1002/1099-0801(200005)14:3<141::aid-bmc937>3.3.co;2-q.
Matsui T, Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol. 2015;27(6):269–80. doi:10.1093/intimm/dxv013.
Acknowledgements
The authors gratefully acknowledge financial support from the National Science and Technology Major Project of the Ministry of Science and Technology of China (Grant No. 2014ZX09507001-009) and the Science and Technology Research Project of Education Department of Liaoning Province (L2013390).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ma, J., Gao, Y., Sun, Y. et al. Tissue distribution and dermal drug determination of indomethacin transdermal-absorption patches. Drug Deliv. and Transl. Res. 7, 617–624 (2017). https://doi.org/10.1007/s13346-017-0392-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0392-5