Abstract
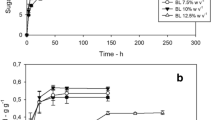
A huge amount of lignocellulosic agro-food processing wastes (AFWs) is produced by agricultural practices and food industries. Coffee silverskin (CSS) is an AFW produced during the coffee bean–roasting process. Pretreatment methods are required to promote the enzymatic hydrolysis of AFWs, including CSS, aimed at their use as feedstock in a sugar-based biorefinery. A combined pretreatment, based on ultrasound and mild alkaline solution, has been optimized for CSS. The effects of sonication time, biomass loading, NaOH concentration and alkaline pretreatment residence time were investigated according to the response surface methodology. The maximum sugar yield (YS = 0.6 g g sugars in pretreated CSS−1) was obtained after enzymatic hydrolysis of CSS pretreated with 5-min sonication at 11% w v−1 biomass loading, and 75-min autoclave in 5% w v−1 NaOH. Fermentation inhibitors in the pretreatment solvent were absent or present at concentrations not affecting the growth of Clostridium sp. relevant for biofuel production. The phenolic content was 25 mgGAE graw_CSS−1.





Similar content being viewed by others
References
De Bowmick G, Sarmah AK, Sen R (2018) Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour Technol 247:1144–1154. https://doi.org/10.1016/j.biortech.2017.09.163
Chandel AK, Garlapati VK, Singh AK, Fernandes Antunes FA, da Silva SS (2018) The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Bioresour Technol 264:370–381. https://doi.org/10.1016/j.biortech.2018.06.004
Dahmen N, Lewandowski I, Zibek S, Weidtmann A (2019) Integrated lignocellulosic value chains in a growing bioeconomy: status quo and perspectives. GCB Bioenergy 11:107–117. https://doi.org/10.1111/gcbb.12586
Friedl A (2012) Lignocellulosic biorefinery. Environ Eng Manag J 11:75–79
Stolarski MJ, Krzyzaniak M, Łuczyński M et al (2015) Lignocellulosic biomass from short rotation woody crops as a feedstock for second-generation bioethanol production. Ind Crop Prod 75:66–75. https://doi.org/10.1016/j.indcrop.2015.04.025
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38:522–550. https://doi.org/10.1016/j.pecs.2012.02.002
Morales A, Gullón B, Dávila I, Eibes G, Labidi J, Gullón P (2018) Optimization of alkaline pretreatment for the co-production of biopolymer lignin and bioethanol from chestnut shells following a biorefinery approach. Ind Crop Prod 124:582–592. https://doi.org/10.1016/j.indcrop.2018.08.032
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions - a review. Bioresour Technol 99:6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173. https://doi.org/10.1016/j.jrras.2014.02.003
Procentese A, Raganati F, Olivieri G, Russo ME, de la Feld M, Marzocchella A (2017) Renewable feedstocks for biobutanol production by fermentation. New Biotechnol 39:135–140. https://doi.org/10.1016/j.nbt.2016.10.010
Procentese A, Raganati F, Olivieri G, Russo ME, de la Feld M, Marzocchella A (2019) Agro food wastes and innovative pretreatments to meet biofuel demand in Europe. Chem Eng Technol 42:954–961. https://doi.org/10.1002/ceat.201800459
Procentese A, Raganati F, Navarini L et al (2018) Coffee silverskin as a renewable resource to produce butanol and isopropanol. Chem Eng Trans 64. https://doi.org/10.3303/CET1864024
Niglio S, Procentese A, Russo ME et al (2017) Ultrasound-assisted dilute acid pretreatment of coffee silverskin for biorefinery applications. Chem Eng Trans 57:109. https://doi.org/10.3303/CET1757019
Ballesteros LF, Teixeira JA, Mussatto SI (2014) Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol 7:3493–3503. https://doi.org/10.1007/s11947-014-1349-z
ICO (2017/18) International coffee organization. http://www.ico.org. Accessed November 2018
Didanna HL (2014) A critical review on feed value of coffee waste for livestock feeding. World J Biol Biol Sci 2:72–86
Saenger M, Hartge EU, Werther J, Ogada T, Siagi Z (2001) Combustion of coffee husks. Renew Energy 23:103–121. https://doi.org/10.1016/S0960-1481(00)00106-3
Conde T, Mussatto SI (2016) Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep Biochem Biotechnol 46:406–409. https://doi.org/10.1080/10826068.2015.1084514
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. https://doi.org/10.1021/ie801542g
Carvalheiro F, Duarte LC, Gírio FM (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J Sci Ind Res 67:849–864
Falls M, Sierra-Ramirez R, Holtzapple MT (2011) Oxidative lime pretreatment of dacotah switchgrass. Appl Biochem Biotechnol 165:243–259. https://doi.org/10.1007/s12010-011-9247-6
Velmurugan R, Muthukumar K (2012) Ultrasound-assisted alkaline pretreatment of sugarcane bagasse for fermentable sugar production: optimization through response surface methodology. Bioresour Technol 112:293–299. https://doi.org/10.1016/j.biortech.2012.01.168
Eblaghi M, Niakousari M, Sarshar M, Mesbahi GR (2016) Combining ultrasound with mild alkaline solutions as an effective pretreatment to boost the release of sugar trapped in sugarcane bagasse for bioethanol production. J Food Process Eng 39:273–282. https://doi.org/10.1111/jfpe.12220
Kim I, Han J (2012) Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenergy 46:210–217. https://doi.org/10.1016/j.biombioe.2012.08.024
Luo J, Fang Z, Smith RL (2014) Ultrasound-enhanced conversion of biomass to biofuels. Prog Energy Combust Sci 41:56–93. https://doi.org/10.1016/j.pecs.2013.11.001
Bussemaker MJ, Zhang D (2013) Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind. Eng. Chem. Res 52:3563–3580. https://doi.org/10.1021/ie3022785
Martin C, Klinke HB, Marcet M et al (2014) Study of the phenolic compounds formed during pretreatment of sugarcane bagasse by wet oxidation and steam explosion. Holzforschung 61:483–487. https://doi.org/10.1515/HF.2007.106
Duret X, Fredon E, Masson E et al (2013) Optimization of acid pretreatment in order to increase the phenolic content of Picea abies bark by surface response methodology. BioResources 8:1258–1273
Chiang P, Lee D, Whiteley CG, Huang C (2017) Antioxidant phenolic compounds from Pinus morrisconicola using compressional-puffing pretreatment and water – ethanol extraction: optimization of extraction parameters. J Taiwan Inst Chem Eng 70:7–14. https://doi.org/10.1016/j.jtice.2016.10.010
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and Agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203. https://doi.org/10.1016/j.foodchem.2005.07.042
Trinh LTP, Choi YS, Bae HJ (2018) Production of phenolic compounds and biosugars from flower resources via several extraction 514 processes. Ind Crop Prod 125:261–268. https://doi.org/10.1016/j.indcrop.2018.09.008
Preeti VE, Sandhya SV, Kuttiraja M, Sindhu R, Vani S, Kumar SR, Pandey A, Binod P (2012) An evaluation of chemical pretreatment methods for improving enzymatic saccharification of chili postharvest residue. Appl Biochem Biotechnol 167:1489–1500. https://doi.org/10.1007/s12010-012-9591-1
Sindhu R, Binod P, Pandey A (2016) A novel sono-assisted acid pretreatment of chili post harvest residue for bioethanol production. Bioresour Technol 213:58–63. https://doi.org/10.1016/j.biortech.2016.02.079
Sluiter A, Hames B, Ruiz R, et al (2012) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure (LAP); NREL/TP-510-42618
McIntosh S, Vancov T (2011) Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 35:3094–3103. https://doi.org/10.1016/j.biombioe.2011.04.018
Adney B, Baker J (2008) Measurement of cellulase activities. Laboratory Analytical Procedure (LAP); NREL; TP 510 42628
Wood IP, Elliston A, Ryden P, Bancroft I, Roberts IN, Waldron KW (2012) Rapid quantification of reducing sugars in biomass hydrolysates: improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenergy 44:117–121. https://doi.org/10.1016/j.biombioe.2012.05.003
Rattanachitthawat S, Suwannalert P, Riengrojpitak S et al (2010) Phenolic content and antioxidant activities in red unpolished Thai rice prevents oxidative stress in rats. J Med Plants Res 4:796–801. https://doi.org/10.5897/JMPR10.067
Montavon P, Kukic KR, Bortlik K (2007) A simple method to measure effective catalase activities: optimization, validation, and application in green coffee. Anal Biochem 360:207–215. https://doi.org/10.1016/j.ab.2006.10.035
Segal LC, Creely J, Martin AEJ, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. J Text Res 29:786–794. https://doi.org/10.1177/004051755902901003
Rehman MSU, Kim I, Chisti Y, Han J (2013) Use of ultrasound in the production of bioethanol from lignocellulosic biomass. Energy Educ Sci Technol 30:1391–1410
Soto-Alvarez C, López Miranda J, Rosales-Castro M, Perez-Verdin G, Rodríguez Pérez MA, Hernández I (2013) Alkaline pretreatment of Mexican pine residues for bioethanol production. Afr J Biotechnol 12:4956–4965. https://doi.org/10.5897/AJB2013.12461
Procentese A, Raganati F, Olivieri G, Russo ME, Marzocchella A (2019) Combined antioxidant-biofuel production from coffee silverskin. Appl Microbiol Biotechnol 103:1021–1029. https://doi.org/10.1007/s00253-018-9530-3
Niglio S, Procentese A, Russo ME, Sannia G, Marzocchella A (2019) Investigation of enzymatic hydrolysis of coffee silverskin aimed at the production of butanol and succinic acid by fermentative processes. BioEnerg Res 12:312–324. https://doi.org/10.1007/s12155-019-09969-6
Hijosa-Valsero M, Garita-Cambronero J, Paniagua-García AI, Díez-Antolínez R (2018) Biobutanol production from coffee silverskin. Microb Cell Factories 17:154. https://doi.org/10.1186/s12934-018-1002
Mussatto SI, Machado EMS, Carneiro LM, Teixeira JA (2012) Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl Energy 92:763–768. https://doi.org/10.1016/j.apenergy.2011.08.020
Yunus R, Salleh SF, Abdullah N, Biak DRA (2010) Effect of ultrasonic pre-treatment on low temperature acid hydrolysis of oil palm empty fruit bunch. Bioresour Technol 101:9792–9796. https://doi.org/10.1016/j.biortech.2010.07.074
Florence TM (1980) Degradation of protein disulphide bonds in dilute alkali. Biochem J 189:507–520. https://doi.org/10.1042/bj1890507
Xu H, Li B, Mu X (2016) Review of alkali-based pretreatment to enhance enzymatic saccharification for lignocellulosic biomass conversion. Ind Eng Chem Res 55:8691–8705. https://doi.org/10.1021/acs.iecr.6b01907
Bhutto AW, Qureshi K, Harijan K, Abro R, Abbas T, Bazmi AA, Karim S, Yu G (2017) Insight into progress in pre-treatment of lignocellulosic biomass. Energy 122:724–745. https://doi.org/10.1016/j.energy.2017.01.005
Jonsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:1–10. https://doi.org/10.1186/1754-6834-6-16
Ezeji TC, Blaschek HP (2008) Fermentation of dried distillers’ grains and solubles (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour Technol 99:5232–5242. https://doi.org/10.1016/j.biortech.2007.09.032
Niglio S, Procentese A, Russo ME, Piscitelli A, Marzocchella A (2019) Integrated enzymatic pretreatment and hydrolysis of apple pomace in a bubble column bioreactor. Accepted for publication in Biochem Eng J
Narita Y, Inouye K (2012) High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem 135:943–949. https://doi.org/10.1016/j.foodchem.2012.05.078
Kim S, Holtzapple MT (2006) Effect of structural features on enzyme digestibility of corn Stover. Bioresour Technol 97:583–591. https://doi.org/10.1016/j.biortech.2005.03.040
Bak JS, Ko JK, Han YH, Lee BC, Choi IG, Kim KH (2009) Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment. Bioresour Technol 100:1285–1290. https://doi.org/10.1016/j.biortech.2008.09.010
Acknowledgements
Luciano Cortese is gratefully acknowledged for performing scanning electron microscopy analysis.
Funding
The present work has been partially funded by the European Union’s Horizon 2020 research and innovation program with the grant agreement no. 654623 “Waste2Fuels - Sustainable production of next generation biofuels from waste streams.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niglio, S., Procentese, A., Russo, M.E. et al. Combined pretreatments of coffee silverskin to enhance fermentable sugar yield. Biomass Conv. Bioref. 10, 1237–1249 (2020). https://doi.org/10.1007/s13399-019-00498-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00498-y