Abstract
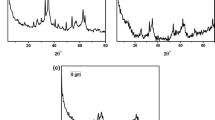
Different silica-based magnetic sorbents including mesoporous silica–Fe3O4 nanocomposite (Fe3O4@MCM-41), amino-functionalized mesoporous silica–Fe3O4 nanocomposite (Fe3O4@MCM-41–NH2) and graphene oxide/amino-functionalized mesoporous silica–Fe3O4 nanocomposite (Fe3O4@MCM-41–NH2–GO) were synthesized and their extraction efficiencies were compared in solid-phase extraction procedure of important parabens prior to gas chromatography–flame ionization detection. The sorbents extraction efficiencies followed the order of Fe3O4@MCM-41–NH2 > Fe3O4@MCM-41–NH2-GO > Fe3O4@MCM-41. Parameters affecting SPE procedure including amount of sorbent, eluting solvent type, adsorption and desorption times, and ionic strength were studied and optimized. The method’s performance was studied in terms of accuracy, linearity, precision and limits of detection. Detection limits of the method were <0.9 µg L−1 and linearity was in the range of 10–1000 µg L−1. Finally, the applicability of the proposed method was demonstrated for extraction of selected parabens in some cosmetic products. Good quantitative recoveries for spiked samples (85–107 %) and satisfactory precision (RSD ≤ 5.6 %) were also obtained.







Similar content being viewed by others
References
V. Čiuvašovaite, E. Adomavičiūtė, V. Vičkačkaitė, Solid-phase microextraction of parabens by polyaniline–polypyrrole coating. Chemija 18, 11–15 (2007)
G. Shanmugam, B.R. Ramaswamy, V. Radhakrishnan, H. Tao, GC-MS method for the determination of paraben preservatives in the human breast cancerous tissue. Microchem. J. 96, 391–396 (2010)
I. Gonzalez-Mariño, J.B. Quintana, I. Rodriguez, S. Schrader, M. Moeder, Fullyautomated determination of parabens, triclosan and methyl triclosan inwastewater by microextraction by packed sorbents and gas chromatography-mass spectrometry. Anal. Chim. Acta 684, 59–66 (2011)
S. Prijic, G. Sersa, Magnetic nanoparticles as targeted delivery systems in oncology. Mag. Radiol. Oncol. 45, 1–16 (2011)
S.P. Gubin, Y.A. Koksharov, G.B. Khomutov, G.Y. Yurkov, Magnetic nanoparticles: preparation, structure and properties. Russ. Chem. Rev. 74, 489–520 (2005)
S.B. Bucak, Yavuztürk, A. DemirSezer, in Recent Advances in Novel Drug Carrier Systems, ed. By A.D. Sezer (InTech, Rijeka, 2012), p. 165
J.K. Xu, C.X. Ju, J. Sheng, F. Wang, Q. Zhang, G.L. Sun, M. Sun, Synthesis and characterization of magnetic nanoparticles and its application in lipase immobilization. Bull. Korean Chem. Soc. 34, 2408–2412 (2013)
L. Chen, T. Wang, J. Tong, Application of derivatized magnetic materials to the separation and the preconcentration of pollutants in water. Trend. Anal. Chem. 30, 1095–1108 (2011)
X.S. Li, G.T. Zhu, Y.B. Luo, B.F. Yuan, Y.Q. Feng, Synthesis and applications of functionalized magnetic materials in sample preparation. Trend. Anal. Chem. 45, 233–247 (2013)
A. Walcarius, C. Delacôte, Mercury(II) binding to thiol-functionalized mesoporous silicas: critical effect of pH and sorbent properties on capacity and selectivity. Anal. Chim. Acta 547, 3–13 (2005)
Z.A. ALOthman, A review: fundamental aspects of silicate mesoporous materials. Materials 5, 2874–2902 (2012)
K. Aguilar-Arteagav, J.A. Rodriguez, E. Barrado, Magnetic solids in analytical chemistry: a review. Anal. Chim. Acta 674, 157–165 (2010)
M. Mahdavi, M.B. Ahmad, M.J. Haron, Y. Gharayebi, K. Shameli, B. Nadi, Fabrication and characterization of SiO2/(3-Aminopropyl) triethoxysilane-coated magnetite nanoparticles for Lead(II) removal from aqueous solution. J. Inorg. Organomet. Polym. 23, 599–607 (2013)
S. Zhang, Z. Du, G. Li, Layer-by-layer fabrication of chemical-bonded graphene coating for solid-phase microextraction. Anal. Chem. 83, 7531–7541 (2011)
E. Tahmasebi, Y. Yamini, A. Mehdinia, F. Rouhi, Polyaniline-coated Fe3O4 nanoparticles: an anion exchange magnetic sorbent for solid-phase extraction. J. Sep. Sci. 35, 2256–2265 (2012)
A. Mehdinia, R. Esfandiarnejad, A. Jabbari, Magnetic nanocomposite of self-doped polyaniline–graphene as a novel sorbent for solid-phase extraction. J. Sep. Sci. 38, 141–147 (2015)
Y.P. Chin, S. Mohamad, M.R.B. Abas, Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer. Int. J. Mol. Sci. 11, 3459–3471 (2011)
M. Abbasghorbani, A. Attaran, M. Payehghadr, Solvent-assisted dispersive micro-SPE by using aminopropyl-functionalized magnetite nanoparticle followed by GC-PID for quantification of parabens in aqueous matrices. J. Sep. Sci. 36, 311–319 (2013)
S. Rostamizadeh, M. Azad, N. Shadjou, M. Hasanzadeh, (α-Fe2O3)-MCM-41-SO3H as a novel magnetic nanocatalyst for the synthesis of N-aryl-2-amino-1,6-naphthyridine derivatives. Catal. Commun. 25, 83–91 (2012)
J.W.S. Hummers, R.E. Offeman, Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958)
N.I. Kovtyukhova, P.J. Ollivier, B.R. Martin, T.E. Mallouk, S.A. Chizhik, E.V. Buzaneva, A.D. Gorchinskiy, Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 11, 771–778 (1999)
A. Prichodko, E. Janenaite, V. Smitiene, V. Vickackaite, Gas chromatographic determination of parabens after in situ derivatization and dispersive liquid-liquid microextraction. Acta Chromatogr. 24, 589–601 (2012)
C. Roy, J. Chakrabarty, Quality by design-based development of a stability-indicating RP-HPLC method for the simultaneous determination of methylparaben, propylparaben, diethylamino hydroxybenzoyl hexyl benzoate, and octinoxate in topical pharmaceutical formulation. Sci. Pharm. 82, 519–539 (2014)
H. Parham, S. Saeed, Ultrasound-assisted solid phase extraction of nitro- and chloro (phenols) using magnetic iron oxide nanoparticles and Aliquat 336 ionic liquid. J. Chromatogr. A 1336, 34–42 (2014)
I. Marquez-Sillero, E. Aguilera-Herrador, S. Cardenas, M. Valcarcel, Determination of parabens in cosmetic products using multi-walled carbon nanotubes as solid phase extraction sorbent and corona-charged aerosol detection system. J. Chromatogr. A 1217, 1–6 (2010)
S.Y. Lee, E. Son, J.Y. Kang, H.S. Lee, M.K. Shin, H.S. Nam, S.Y. Kim, Y.M. Jang, G.S. Rhee, Development of a quantitative analytical method for determining the concentration of human urinary paraben by LC-MS/MS. Bull. Korean Chem. Soc. 34, 1131–1136 (2013)
L. Wang, X. Zang, C. Wang, Z. Wang, Graphene oxide as a micro-solid-phase extraction sorbent for the enrichment of parabens from water and vinegar samples. J. Sep. Sci. 37, 1656–1662 (2014)
Acknowledgments
We gratefully acknowledge the financial support of this work by the Iranian National Science Foundation with grant number 92001925.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehdinia, A., Bahrami, M. & Mozaffari, S. A comparative study on different functionalized mesoporous silica nanomagnetic sorbents for efficient extraction of parabens. J IRAN CHEM SOC 12, 1543–1552 (2015). https://doi.org/10.1007/s13738-015-0626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0626-8