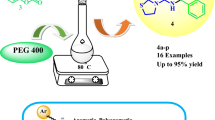
Abstract
A new three-component reaction between salicylaldehydes, barbituric acid, pyrazolones, in the presence of para-toluenesulfonic acid, efficiently provides pyrazol-chromeno[2,3-d]pyrimidine-ones derivatives in good yields in ethanol/water at 70 °C. This multicomponent reaction showed high atom economy.
Graphical Abstract



Similar content being viewed by others
References
Multicomponent Reactions ed. by J. Zhu, H. Bienaymé (Wiley, Weinheim, 2005)
Domino Reactions in Organic Synthesis ed. by L.F. Tietze, G. Brasche, K. Gericke (Wiley, Weinheim, 2006)
U. Dömling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168–3210 (2000)
E. Soleimani, M.M. Khodaei, N. Batooie, M. Baghbanzadeh, Green Chem. 13, 566–569 (2011)
E. Soleimani, M. Zainali, J. Org. Chem. 76, 10306–10311 (2011)
E. Soleimani, M. Zainali, S. Samadi, Tetrahedron Lett. 52, 4186–4188 (2011)
E. Soleimani, M. Zainali, N. Ghasemi, B. Notash, Tetrahedron 69, 9832–9838 (2013)
B.S. Holla, M. Mahalinga, M.S. Karthikeyan, P.M. Akberali, N.S. Shetty, Bioorg. Med. Chem. 14, 2040–2047 (2006)
A.H. Shamroukh, M.E.A. Zaki, E.M.H. Morsy, F.M. Abdel-Motti, F.M.E. Abdel-Megeid, Arch. Pharm. Chem. Life Sci. 14, 340–345 (2007)
E. Akbas, I. Berber, Eur. J. Med. Chem. 40, 401–405 (2005)
R.Z. Yan, X.Y. Liu, W.F. Xu, C. Pannecouque, M. Witvrouw, E. DeClercq, Arch. Pharm. Res. 29, 957–962 (2006)
L.C. Chou, L.J. Huang, J.S. Yang, F.Y. Lee, C.M. Teng, S.C. Kuo, Bioorg. Med. Chem. 15, 1732–1740 (2007)
J. Li, Y.F. Zhao, X.L. Zhao, X.Y. Yuan, P. Gong, Arch. Pharm. Chem. Life Sci. 339, 593–597 (2006)
V. Krystof, D. Moravcova, M. Paprskarova, P. Barbier, V. Peyrot, A. Hlobilkova, L. Havlicek, M. Strnad, Eur. J. Med. Chem. 41, 1405–1411 (2006)
S. Schenone, O. Bruno, A. Ranise, F. Bondavalli, C. Brullo, P. Fossa, L. Mosti, G. Menozzi, F. Carraro, A. Naldini, C. Bernini, F. Manettic, M. Botta, Bioorg. Med. Chem. Lett. 14, 2511–2517 (2004)
G. Daidone, D. Raffa, B. Maggio, M.V. Raimondi, F. Plescia, D. Schillaci, Eur. J. Med. Chem. 39, 219–224 (2004)
S.C. Ren, F.Y. Sheau, M.L. Chih, G. Amooru, T.K. Damu, C.P. Cheng, F.B. Kenneth, L.K. Hsiung, W.T. Shung, Bioorg. Med. Chem. 17, 6137–6143 (2009)
A.V. Karnik, A.M. Kulkarni, N.J. Malviya, B.R. Mourya, B.L. Jadhav, Eur. J. Med. Chem. 43, 2615–2617 (2008)
K.J. Hwan, K.H. Eun, J.J. Kyung, K. Hwajung, C. Jungsook, L. Heesoon, Arch. Pharm. Res. 29, 728–734 (2006)
C. Fakher, M. Mehdi, B.M. Hedi, C. Leila, S. Mansour, Eur. J. Med. Chem. 42, 715–718 (2007)
C.Y. Cheng, H. Chiu, M.J. Chang, Y.C. Lin, M.C. Tsai, H.C. Yu, Bioorg. Med. Chem. Lett. 8, 463–468 (1998)
O.A. Abdou, H.E. Ahmed, A.A. Sayed, H.Z. Yasser, J. Sulf. Chem. 26, 405–410 (2005)
M.B. Deshmukh, S.M. Salunkhe, D.R. Patil, P.V. Anbhule, Eur. J. Med. Chem. 44, 2651–2654 (2009)
G. Cecile, D. Douguet, V. Huteau, M. Gilles, M.L. Helene, P. Sylvie, Bioorg. Med. Chem. 16, 6075–6085 (2008)
R. Lin, G. Sigmond, P.J. Johnson, S.K. Connolly, E. Wetter, T.V. Binnun, W.V. Hughes, N.B. Murray, S.J. Pandey, M.M. Mazza, A.R. Adams, F. Pesquera, A.M. Steven, Bioorg. Med. Chem. Lett. 19, 2333–2337 (2009)
E.P. da Silva Falcao, S.J. Melo, R.M. Srivastava, M.T. Catanho, S.C. Nascimento, Eur. J. Med. Chem. 41, 276–282 (2006)
Q. Chen, X. Zhu, L. Jiang, L.M. Yang, G. Fu, Eur. J. Med. Chem. 43, 595–603 (2008)
A. Agarwal, R. Ashutosh, N. Goyal, P.M.S. Chauhan, S. Gupta, Bioorg. Med. Chem. 13, 6678–6684 (2005)
E. Soleimani, S. Ghorbani, H.R. Ghasempour, Tetrahedron 69, 8511–8515 (2013)
X.J. Wang, J. Tan, K. Grozinger, Tetrahedron Lett. 41, 4713–4716 (2000)
Acknowledgments
We gratefully acknowledge financial support from the Iran National Science Foundation (INSF) and Research Council of Razi University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soleimani, E., Ghanbarian, M., Saei, P. et al. Synthesis of new derivatives of pyrazol-chromeno[2,3-d]pyrimidine-ones by a one-pot three-component reaction. J IRAN CHEM SOC 12, 2227–2232 (2015). https://doi.org/10.1007/s13738-015-0701-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0701-1