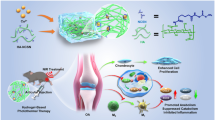
Abstract
Background:
In this study, we have investigated whether human fetal cartilage progenitor cells (hFCPCs) have anti-inflammatory activity and can alleviate osteoarthritis (OA) phenotypes in vitro.
Methods:
hFCPCs were stimulated with various cytokines and their combinations and expression of paracrine factors was examined to find an optimal priming factor. Human chondrocytes or SW982 synoviocytes were treated with interleukin-1β (IL-1β) to produce OA phenotype, and co-cultured with polyinosinic-polycytidylic acid (poly(I-C))-primed hFCPCs to address their anti-inflammatory effect by measuring the expression of OA-related genes. The effect of poly(I-C) on the surface marker expression and differentiation of hFCPCs into 3 mesodermal lineages was also examined.
Results:
Among the priming factors tested, poly(I-C) (1 µg/mL) most significantly induced the expression of paracrine factors such as indoleamine 2,3-dioxygenase, histocompatibility antigen, class I, G, tumor necrosis factor- stimulated gene-6, leukemia inhibitory factor, transforming growth factor-β1 and hepatocyte growth factor from hFCPCs. In the OA model in vitro, co-treatment of poly(I-C)-primed hFCPCs significantly alleviated IL-1β-induced expression of inflammatory factors such as IL-6, monocyte chemoattractant protein-1 and IL-1β, and matrix metalloproteinases in SW982, while it increased the expression of cartilage extracellular matrix such as aggrecan and collagen type II in human chondrocytes. We also found that treatment of poly(I-C) did not cause significant changes in the surface marker profile of hFCPCs, while showed some changes in the 3 lineages differentiation.
Conclusion:
These results suggest that poly(I-C)-primed hFCPCs have an ability to modulate inflammatory response and OA phenotypes in vitro and encourage further studies to apply them in animal models of OA in the future.








Similar content being viewed by others
References
Ishii T, Eto K. Fetal stem cell transplantation: past, present and future. World J Stem Cells. 2014;6:404–20.
Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: do not discard. J Cell Mol Med. 2008;12:730–42.
Shin KS, Na KH, Lee HJ, Kim DG, Shin SJ, Kim JK, et al. Characterization of fetal tissue-derived mesenchymal stem cells. Int J Stem Cells. 2009;2:51–8.
Götherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–45.
Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49.
Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Kao WW. Extrinsic and intrinsic mechanisms by which mesenchymal stem cells suppress the immune system. Ocul Surf. 2016;14:121–34.
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507.
Najar M, Krayem M, Meuleman N, Bron D, Lagneaux L. Mesenchymal stromal cells and toll-like receptor priming: a critical review. Immune Netw. 2017;17:89–102.
Shirjang S, Mansoori B, Solali S, Hagh MF, Shamsasenjan K. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell Immunol. 2017;135:1–10.
Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, et al. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transplant. 2016;25:449–61.
Kim HR, Kim J, Park SR, Min BH, Choi BH. Characterization of human fetal cartilage progenitor cells during long-term expansion in a xeno-free medium. Tissue Eng Regen Med. 2018;15:649–59.
Lee SJ, Kim J, Choi WH, Park SR, Choi BH, Min BH. Immunophenotype and immune-modulatory activities of human fetal cartilage-derived progenitor cells. Cell Transplant. 2019;28:932–42.
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–92.
Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol. 2017;29:79–85.
Hermann W, Lambova S, Muller-Ladner U. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018;14:108–16.
Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040.
Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14:497–507.
Richards MM, Maxwell JS, Weng L, Angelos MG, Golzarian J. Intra-articular treatment of knee osteoarthritis: from anti-inflammatories to products of regenerative medicine. Phys Sportsmed. 2016;44:101–8.
Im GI. Perspective on intra-articular injection cell therapy for osteoarthritis treatment. Tissue Eng Regen Med. 2019;16:357–63.
Hwang JJ, Rim YA, Nam Y, Ju JH. Recent developments in clinical applications of mesenchymal stem cells in the treatment of rheumatoid arthritis and osteoarthritis. Front Immunol. 2021;12:631291.
Kwon DG, Kim MK, Jeon YS, Nam YC, Park JS, Ryu DJ. State of the art: the immunomodulatory role of MSCs for osteoarthritis. Int J Mol Sci. 2022;23:1618.
Iijima H, Isho T, Kuroki H, Takahashi M, Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3:15.
Song Y, Zhang J, Xu H, Lin Z, Chang H, Liu W, et al. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translat. 2020;24:121–30.
Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–21.
Lim HC, Park YB, Ha CW, Cole BJ, Lee BK, Jeong HJ, et al. Allogeneic umbilical cord blood-derived mssenchymal stem cell implantation verus microfracture for large, full-tickness cartilage defects in older patients. Orthop J Sports Med. 2021;9:2325967120973052.
Im GI. The concept of early osteoarthritis and its significance in regenerative medicine. Tissue Eng Regen Med. 2022;19:431–6.
Yan H, Wu M, Yuan Y, Wang ZZ, Jiang H, Chen T. Priming of Toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem Biophys Res Commun. 2014;448:212–7.
Raicevic G, Najar M, Stamatopoulos B, De Bruyn C, Meuleman N, Bron D, et al. The source of human mesenchymal stromal cells influences their TLR profile as well as their functional properties. Cell Immunol. 2011;270:207–16.
Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cells (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088.
Kota DJ, DiCarlo B, Hetz RA, Smith P, Cox CS Jr, Olson SD. Differential MSC activation leads to distinct mononuclear leukocyte binding mechanisms. Sci Rep. 2014;4:4565.
Ryu JH, Park M, Kim BK, Ryu KH, Woo SY. Tonsil-derived mesenchymal stromal cells produce CXCR2-binding chemokines and acquire follicular dendritic cell-like phenotypes under TLR3 stimulation. Cytokine. 2015;73:225–35.
Zhang L, Liu D, Pu D, Wang Y, Li L, He Y, et al. The role of Toll-like receptor 3 and 4 in regulating the function of mesenchymal stem cells isolated from umbilical cord. Int J Mol Med. 2015;35:1003–10.
Lombardo E, DelaRosa O, Mancheño-Corvo P, Menta R, Ramírez C, Büscher D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 2009;15:1579–89.
Raicevic G, Najar M, Pieters K, De Bruyn C, Meuleman N, Bron D, et al. Inflammation and Toll-like receptor ligation differentially affect the osteogenic potential of human mesenchymal stromal cells depending on their tissue origin. Tissue Eng Part A. 2012;18:1410–8.
Romieu-Mourez R, François M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;182:7963–73.
Dumitru CA, Hemeda H, Jakob M, Lang S, Brandau S. Stimulation of mesenchymal stromal cells (MSCs) via TLR3 reveals a novel mechanism of autocrine priming. FASEB J. 2014;28:3856–66.
Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, Peyrafitte JA, et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65:1271–81.
Ichiseki T, Shimazaki M, Ueda Y, Ueda S, Tsuchiya M, Souma D, et al. Intraarticularly-injected mesenchymal stem cells stimulate anti-inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate-induced rat arthritis model. Int J Mol Sci. 2018;19:203.
Corsello T, Amico G, Corrao S, Anzalone R, Timoneri F, Lo Iacono M, et al. Wharton’s Jelly mesenchymal stromal cells from human umbilical cord: a close-up on immunomodulatory molecules featured in situ and in vitro. Stem Cell Rev Rep. 2019;15:900–18.
Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Kim M, Shin DI, Choi BH, Min BH. Exosomes from IL1β -primed mesenchymal stem cells inhibited IL-1β and TNF-α-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng Regen Med. 2021;18:525–36.
Kawakubo K, Ohnishi S, Fujita H, Kuwatani M, Onishi R, Masamune A, et al. Effect of fetal membrane-derived mesenchymal stem cell transplantation in rats with acute and chronic pancreatitis. Pancreas. 2016;45:707–13.
Wu KJ, Yu SJ, Chiang CW, Lee YW, Yen BL, Tseng PC, et al. Neuroprotective action of human Wharton’s jelly-derived mesenchymal stromal cell transplants in a rodent model of stroke. Cell Transplant. 2018;27:1603–12.
Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;17:303–13.
PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–34.
Fettke F, Schumacher A, Canellada A, Toledo N, Bekeredjian-Ding I, Bondt A, et al. Maternal and fetal mechanisms of B cell regulation during pregnancy: human chorionic gonadotropin stimulates B cells to produce IL-10 while alpha-fetoprotein drives them into apoptosis. Front Immunol. 2016;7:495.
Rebmann V, König L, Nardi Fda S, Wagner B, Manvailer LF, Horn PA. The potential of HLA-G-bearing extracellular vesicles as a future element in HLA-G immune biology. Front Immunol. 2016;7:173.
Xu YY, Wang SC, Li DJ, Du MR. Co-signaling molecules in maternal-fetal immunity. Trends Mol Med. 2017;23:46–58.
Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA-G: at the interface of maternal-fetal tolerance. Trends Immunol. 2017;38:272–86.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (HI17C2191) and the National Research Foundation Grant (NRF-2019M3E5D1A02070861).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical statement
The experiments were conducted with the approval of institutional review board (IRB) of Ajou University Medical Center (AJIRB-CRO-07-139) and (AJIRB-MED-SMP-10–266).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J., Tran, A.NT., Lee, J.Y. et al. Human Fetal Cartilage-Derived Progenitor Cells Exhibit Anti-Inflammatory Effect on IL-1β-Mediated Osteoarthritis Phenotypes In Vitro. Tissue Eng Regen Med 19, 1237–1250 (2022). https://doi.org/10.1007/s13770-022-00478-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00478-w