Abstract
Introduction
Drug-induced torsades de pointes (TdP) is a potentially lethal ventricular arrhythmia that is associated with drugs that prolong the QT interval on the electrocardiogram (ECG) due to their interference with the cardiac potassium current, IKR. Intravenous (IV) formulations of methadone have been associated with TdP and contain the preservative chlorobutanol, which, like methadone, blocks IKR. The combinations of chlorobutanol with methadone or terfenadine, another IKR blocker, produce synergistic IKR block.
Objective
The aim of this study was to examine and summarize the evidence available to address the question: what other IV drug formulations contain chlorobutanol and are they associated with TdP?
Methods
IV drug products containing the preservative chlorobutanol were identified by searching the websites DailyMed (https://dailymed.nlm.nih.gov/dailymed/index.cfm) and Drugs@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). For each drug identified, PubMed and the FDA’s Adverse Event Reporting System (FAERS) were searched for reports of TdP and/or QT prolongation and FAERS data were analyzed for disproportionality of reports.
Results
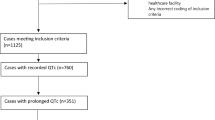
The search found nine drugs (methadone, epinephrine, papaverine, oxytocin, vasopressin, testosterone, estradiol, isoniazid, and desmopressin) that contain chlorobutanol 2.5 (n = 1) or 5.0 mg/mL. All nine drugs had reports of QT prolongation or TdP reported in FAERS and all but estradiol, testosterone, desmopressin, and isoniazid had reports of QT prolongation or TdP in PubMed. Two of the nine drugs (epinephrine and methadone) had positive signals (by disproportionality analysis) for TdP in FAERS (EB05 2.88 and 23.81, respectively) and four (methadone, epinephrine, papaverine, and vasopressin) were reported in published articles as the suspect drugs in cases of TdP.
Conclusion
The pharmacologic profile of chlorobutanol (synergistic IKR block) and its association with reports of TdP and QT prolongation suggest the need for a full evaluation of its cardiac safety when used as a preservative in IV drug and vitamin formulations.
Similar content being viewed by others
References
Roden DM. Predicting drug-induced QT prolongation and torsades de Pointes. J Physiol. 2015. https://doi.org/10.1113/jp270526.
Woosley R, Romero K, Heise C, Gallo T, Tate J, Woosley R, et al. Adverse Drug Event Causality Analysis: a process for evaluating evidence and assigning drugs to risk categories for sudden death. Drug Saf. 2017;40(6):465–74. https://doi.org/10.1007/s40264-017-0519-0.
Woosley RL, Heise CW, Gallo T, Tate J, Woosley D, Romero KA. CredibleMeds. 2019. https://www.crediblemeds.org. Accessed 03 Jan 2019.
Woosley RL, Black K, Heise CW, Romero K. CredibleMedsorg: what does it offer? Trends Cardiovasc Med. 2018;28(2):94–9. https://doi.org/10.1016/j.tcm.2017.07.010.
Katchman AN, Ebert SN, McCroary KA, Woosley RL. Methadone block HERG current in transfected HEK cells. Pharmacologist. 2001;43:98.
Katchman AN, McGroary KA, Kilborn MJ, Kornick CA, Manfredi PL, Woosley RL, et al. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exp Ther. 2002;303(2):688–94. https://doi.org/10.1124/jpet.102.038240.
Kornick CA, Kilborn MJ, Santiago-Palma J, Schulman G, Thaler HT, Keefe DL, et al. QTc interval prolongation associated with intravenous methadone. Pain. 2003;105(3):499–506. https://doi.org/10.1016/s0304-3959(03)00205-7.
Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137(6):501–4.
Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14(11):747–53. https://doi.org/10.1002/pds.1112.
Friemel A, Zunkler BJ. Interactions at human ether-a-go-go-related gene channels. Toxicol Sci. 2010;114(2):346–55. https://doi.org/10.1093/toxsci/kfq011.
Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269(12):1532–6.
Drugs@FDA. U.S. Food and Drug Administration. 2017. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 15 Oct 2017.
DailyMed. US National Library of Medicine. 2019. https://dailymed.nlm.nih.gov/dailymed/search.cfm?adv=1&labeltype=all&pagesize=200&page=1&query=%28chlorobutanol%29+. Accessed 15 Oct 2017.
European Public Assessment Reports. European Medicines Agency, European Union. 2017. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124. Accessed 15 Oct 2017.
Australian Register of Therapeutic Goods. Government of Australia. 2017. http://tga-search.clients.funnelback.com/s/search.html?query=&collection=tga-artg. Accessed 15 Oct 2017.
Japanese Drug Products. Japanese Pharmaceuticals and Medical Device Agency. 2017. https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html. Accessed 15 Oct 2017.
Canadian Drug Product Database. Government of Canada, Health Canada. 2017. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html. Accessed 15 Oct 2017.
QHTS Assay for Small Molecule Inhibitors of the Human HERG Channel Activity. U.S. Government, PubChem. 2017. https://pubchem.ncbi.nlm.nih.gov/bioassay/588834. Accessed 15 Oct 2017.
Almenoff JS, DuMouchel W, Kindman LA, Yang X, Fram D. Disproportionality analysis using empirical Bayes data mining: a tool for the evaluation of drug interactions in the post-marketing setting. Pharmacoepidemiol Drug Saf. 2003;12(6):517–21.
Vyas H, Hejlik J, Ackerman MJ. Epinephrine QT stress testing in the evaluation of congenital long-QT syndrome: diagnostic accuracy of the paradoxical QT response. Circulation. 2006;113(11):1385–92. https://doi.org/10.1161/CIRCULATIONAHA.105.600445.
Klein GJ. Vasopressin, “torsades de pointes”, and QT syndrome. Ann Intern Med. 1980;93(3):511–2.
Stein LB, Dabezies MA, Silverman M, Brozena SC. Fatal torsade de pointes occurring in a patient receiving intravenous vasopressin and nitroglycerin. J Clin Gastroenterol. 1992;15(2):171–4.
Faigel DO, Metz DC, Kochman ML. Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalance. Am J Gastroenterol. 1995;90(5):822–4.
Kelly KJ, Stang JM, Mekhjian HS. Vasopressin provocation of ventricular dysrhythmia. Ann Intern Med. 1980;92(2 Pt 1):205–6.
Mauro VF, Bingle JF, Ginn SM, Jafri FM. Torsade de pointes in a patient receiving intravenous vasopressin. Crit Care Med. 1988;16(2):200–1.
Turner S, Mathews L, Pandharipande P, Thompson R. Dolasetron-induced torsades de pointes. J Clin Anesth. 2007;19(8):622–5. https://doi.org/10.1016/j.jclinane.2007.03.012.
Jain A, Jenkins MG. Intracoronary electrocardiogram during torsade des pointes secondary to intracoronary papaverine. Catheter Cardiovasc Diagn. 1989;18(4):255–7.
Kern MJ, Deligonul U, Serota H, Gudipati C, Buckingham T. Ventricular arrhythmia due to intracoronary papaverine: analysis of QT intervals and coronary vasodilatory reserve. Catheter Cardiovasc Diagn. 1990;19(4):229–36.
Vrolix M, Piessens J, De GH. Torsades de pointes after intracoronary papaverine. Eur Heart J. 1991;12(2):273–6.
Nakayama M, Saito A, Kitazawa H, Takahashi M, Sato M, Fuse K, et al. Papaverine-induced polymorphic ventricular tachycardia in relation to QTU and giant T-U waves in four cases. Intern Med. 2012;51(4):351–6. https://doi.org/10.2169/internalmedicine/51.6567.
Goto M, Sato M, Kitazawa H, Takahashi M, Fuse K, Saito A, et al. Papaverine-induced QT interval prolongation and ventricular fibrillation in a patient with a history of drug-induced QT prolongation. Intern Med. 2014;53(15):1629–31. https://doi.org/10.2169/internalmedicine/53.1828.
Nakayama M, Tanaka N, Sakoda K, Hokama Y, Hoshino K, Kimura Y, et al. Papaverine-induced polymorphic ventricular tachycardia during coronary flow reserve study of patients with moderate coronary artery disease. Circ J. 2015;79(3):530–6. https://doi.org/10.1253/circj.CJ-14-1118.
Inoue F, Hashimoto T, Fujimoto S, Uemura S, Kawamoto A, Dohi K. Estimation of coronary flow reserve by intracoronary administration of nicorandil: comparison with intracoronary administration of papaverine. Heart Vessels. 1998;13(5):229–36.
Inoue T, Asahi S, Takayanagi K, Morooka S, Takabatake Y. QT prolongation and possibility of ventricular arrhythmias after intracoronary papaverine. Cardiology. 1994;84(1):9–13. https://doi.org/10.1159/000176323.
Zhang X, Shen W, Cai X, Zheng A. Polymorphous ventricular tachycardia after intracoronary papaverine: a report of 3 cases. Chin Med Sci J. 1993;8(4):248–9.
Christensen CW, Rosen LB, Gal RA, Haseeb M, Lassar TA, Port SC. Coronary vasodilator reserve. Comparison of the effects of papaverine and adenosine on coronary flow, ventricular function, and myocardial metabolism. Circulation. 1991;83(1):294–303.
Talman CL, Winniford MD, Rossen JD, Simonetti I, Kienzle MG, Marcus ML. Polymorphous ventricular tachycardia: a side effect of intracoronary papaverine. J Am Coll Cardiol. 1990;15(2):275–8.
Okabe Y, Otowa K, Mitamura Y, Murai H, Usui S, Kaneko S, et al. Evaluation of the risk factors for ventricular arrhythmias secondary to QT prolongation induced by papaverine injection during coronary flow reserve studies using a 4 Fr angio-catheter. Heart Vessels. 2018. https://doi.org/10.1007/s00380-018-1175-8.
Charbit B, Funck-Brentano C, Samain E, Jannier-Guillou V, Albaladejo P, Marty J. QT interval prolongation after oxytocin bolus during surgical induced abortion. Clin Pharmacol Ther. 2004;76(4):359–64. https://doi.org/10.1016/j.clpt.2004.06.005.
Liou SC, Chen C, Wong SY, Wong KM. Ventricular tachycardia after oxytocin injection in patients with prolonged Q-T interval syndrome–report of two cases. Acta Anaesthesiol Sin. 1998;36(1):49–52.
Richardson MG, Roark GL, Helfaer MA. Intraoperative epinephrine-induced torsades de pointes in a child with long QT syndrome. Anesthesiology. 1992;76(4):647–9.
Kim JH, Park SH, Kim KH, Choi WS, Kang JK, Kim NY, et al. Epinephrine-induced polymorphic ventricular tachycardia in a patient with congenital long QT syndrome. Korean Circ J. 2009;39(9):386–8. https://doi.org/10.4070/kcj.2009.39.9.386.
Tan VH, Duff H, Kuriachan V, Gerull B. Congenital long QT syndrome: severe torsades de pointes provoked by epinephrine in a digenic mutation carrier. Heart Lung. 2014;43(6):541–5. https://doi.org/10.1016/j.hrtlng.2014.07.004.
Young JB, Vandermolen LA, Pratt CM. Torsade de pointes: an unusual manifestation of chloral hydrate poisoning. Am Heart J. 1986;112(1):181–4.
Sing K, Erickson T, Amitai Y, Hryhorczuk D. Chloral hydrate toxicity from oral and intravenous administration. J Toxicol Clin Toxicol. 1996;34(1):101–6.
Brun PM, Querellou E, Leyral J, Barberis C, Levy D, Puidupin A. Chlorobutanol poisoning: about a case. Ann Fr Anesth Reanim. 2010;29(10):741–2. https://doi.org/10.1016/j.annfar.2010.06.014.
Valentour JC, Sunshine I. Chlorobutanol poisoning. Report of a fatal case. Z Rechtsmed. 1975;77(1):61–3.
Nordt SP. Chlorobutanol toxicity. Ann Pharmacother. 1996;30(10):1179–80.
DeChristoforo R, Corden BJ, Hood JC, Narang PK, Magrath IT. High-dose morphine infusion complicated by chlorobutanol-induced somnolence. Ann Intern Med. 1983;98(3):335–6.
Smith WS, Dowd CF, Johnston SC, Ko NU, DeArmond SJ, Dillon WP, et al. Neurotoxicity of intra-arterial papaverine preserved with chlorobutanol used for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2004;35(11):2518–22. https://doi.org/10.1161/01.STR.0000144682.00822.83.
Hofmann H, Goerz G, Plewig G. Anaphylactic shock from chlorobutanol-preserved oxytocin. Contact Dermat. 1986;15(4):241.
Lian TS, Bellan JA, Jahr JS, Holton RH, Nossaman BD, Kadowitz PJ. Vasodilator responses to desmopressin and its diluent, chlorobutanol, in the hindquarters vascular bed of the cat. J Med. 1996;27(1–2):73–87.
Itabashi A, Katayama S, Yamaji T. Hypersensitivity to chlorobutanol in DDAVP solution. Lancet. 1982;1(8263):108.
Hermsmeyer K, Aprigliano O. Effects of chlorobutanol and bradykinin on myocardial excitation. Am J Physiol. 1976;230(2):306–10. https://doi.org/10.1152/ajplegacy.1976.230.2.306.
List of Withdrawn Drugs. Wikipedia. https://en.wikipedia.org/wiki/List_of_withdrawn_drugs. Accessed 24 Oct 2017.
Molokhia M, Pathak A, Lapeyre-Mestre M, Caturla L, Montastruc JL, McKeigue P. Case ascertainment and estimated incidence of drug-induced long-QT syndrome: study in Southwest France. Br J Clin Pharmacol. 2008;66(3):386–95. https://doi.org/10.1111/j.1365-2125.2008.03229.x.
Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace. 2014;16(1):101–8. https://doi.org/10.1093/europace/eut214.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047–60.
Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88(4):315–25. https://doi.org/10.1016/j.mayocp.2013.01.013.
Sorita A, Bos JM, Morlan BW, Tarrell RF, Ackerman MJ, Caraballo PJ. Impact of clinical decision support preventing the use of QT-prolonging medications for patients at risk for torsade de pointes. J Am Med Inform Assoc. 2015;22(e1):e21–7. https://doi.org/10.1136/amiajnl-2014-002896.
Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–87. https://doi.org/10.1161/CIRCOUTCOMES.113.000152.
Tisdale JE, Jaynes HA, Kingery JR, Overholser BR, Mourad NA, Trujillo TN, et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014;7(3):381–90. https://doi.org/10.1161/CIRCOUTCOMES.113.000651.
Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017;39(1):16–25. https://doi.org/10.1007/s11096-016-0414-2.
Letsas KP, Efremidis M, Kounas SP, Pappas LK, Gavrielatos G, Alexanian IP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98(4):208–12. https://doi.org/10.1007/s00392-008-0741-y.
Vlachos K, Georgopoulos S, Efremidis M, Sideris A, Letsas KP. An update on risk factors for drug-induced arrhythmias. Expert Rev Clin Pharmacol. 2016;9(1):117–27. https://doi.org/10.1586/17512433.2016.1100073.
Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine. 2003;82(4):282–90. https://doi.org/10.1097/01.md.0000085057.63483.9b.
Stockbridge N, Zhang J, Garnett C, Malik M. Practice and challenges of thorough QT studies. J Electrocardiol. 2012;45(6):582–7. https://doi.org/10.1016/j.jelectrocard.2012.07.020.
Johannesen L, Vicente J, Gray RA, Galeotti L, Loring Z, Garnett CE, et al. Improving the assessment of heart toxicity for all new drugs through translational regulatory science. Clin Pharmacol Ther. 2014;95(5):501–8. https://doi.org/10.1038/clpt.2013.238.
Simons FE. First-aid treatment of anaphylaxis to food: focus on epinephrine. J Allergy Clin Immunol. 2004;113(5):837–44. https://doi.org/10.1016/j.jaci.2004.01.769.
Yamazaki D, Kitaguchi T, Ishimura M, Taniguchi T, Yamanishi A, Saji D, et al. Proarrhythmia risk prediction using human induced pluripotent stem cell-derived cardiomyocytes. J Pharmacol Sci. 2018. https://doi.org/10.1016/j.jphs.2018.02.005.
Acknowledgements
The authors gratefully acknowledge the contributions of Kristin Black and Marius Petriu for technical maintenance of the http://www.CredibleMeds.org website and the QTdrugs knowledge base.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors, R. David Woosley, Klaus Romero, Craig W. Heise, Tyler Gallo, Jared Tate, and Raymond L. Woosley have no conflicts of interest that are directly relevant to the content of this work.
Funding
This research was supported by award number HHSF223201400189C from the Safe Use Initiative of the US Food and Drug Administration and the Bert W. Martin Foundation.
Ethical Approval
Not applicable.
Patient Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Woosley, R.D., Romero, K., Heise, C.W. et al. Summary of Torsades de Pointes (TdP) Reports Associated with Intravenous Drug Formulations Containing the Preservative Chlorobutanol. Drug Saf 42, 907–913 (2019). https://doi.org/10.1007/s40264-019-00804-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-019-00804-7