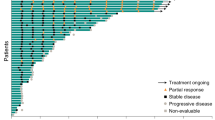
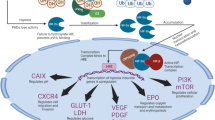
Abstract
Belzutifan (Welireg™) is an oral small molecule inhibitor of hypoxia-inducible factor (HIF)-2α being developed by Peloton Therapeutics for the treatment of solid tumours, including renal cell carcinoma (RCC) with clear cell histology (ccRCC) and von Hippel–Lindau (VHL) disease-associated RCC. In August 2021, belzutifan received its first approval in the USA for the treatment of patients with VHL disease who require therapy for associated RCC, central nervous system (CNS) haemangioblastomas or pancreatic neuroendocrine tumours (pNET), not requiring immediate surgery. Clinical studies of belzutifan (as monotherapy or combination therapy) in other indications, including ccRCC, pNET and phaeochromocytoma/paraganglioma, are also underway in various countries. This article summarizes the milestones in the development of belzutifan leading to this first approval for certain VHL disease-associated tumours.
Similar content being viewed by others
References
Chung C. From oxygen sensing to angiogenesis: targeting the hypoxia signaling pathway in metastatic kidney cancer. Am J Health Syst Pharm. 2020;77(24):2064–73.
Yu Y, Yu Q, Zhang X. Allosteric inhibition of HIF-2α as a novel therapy for clear cell renal cell carcinoma. Drug Discov Today. 2019;24(12):2332–40.
Schonberger T, Fandrey J, Prost-Fingerle K. Ways into understanding HIF inhibition. Cancers. 2021;13(1):1–16.
Merck Sharp & Dohme Corp. Welireg™ (belzutifan) tablets, for oral use: US prescribing information. 2021. https://www.accessdata.fda.gov/. Accessed 24 Aug 2021.
National Organization for Rare Disorders. Von Hippel-Lindau disease. 2021. https://rarediseases.org/rare-diseases/von-hippel-lindau-disease/. Accessed 24 Aug 2021.
Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19(6):617–23.
Kotecha RR, Motzer RJ, Voss MH. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol. 2019;16(10):621–33.
Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14(15):4726–34.
Merck & Co. FDA approves Merck’s hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor Welireg™ (belzutifan) for the treatment of patients with certain types of von Hippel-Lindau (VHL) disease-associated tumors [media release]. 13 Aug 2021. https://www.merck.com.
Merck AG. Merck to acquire Peloton Therapeutics, bolstering oncology pipeline [media release]. 22 May 2019. http://www.merck.com.
Merck C. Merck announces second-quarter 2019 financial results [media release]. 31 Jul 2019. http://www.merck.com.
Merck & Co Inc. Form 10-K. https://www.sec.gov/ix?doc=/Archives/edgar/data/310158/000031015821000004/mrk-20201231.htm. Accessed 24 Aug 2021.
Xu R, Wang K, Rizzi JP, et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible factor 2α (HIF-2α) inhibitor for the treatment of clear cell renal cell carcinoma. J Med Chem. 2019;62(15):6876–93.
Ghosh MC, Zhang DL, Ollivierre WH, et al. Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases. Blood. 2021;137(18):2509–19.
Suresh S, Rajvanshi PK, Noguchi CT. The many facets of erythropoietin physiologic and metabolic response. Front Physiol. 2019;10:1534.
Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med. 2021;27(5):802–5.
Srinivasan R, Donskov F, Iliopoulos O, et al. Phase 2 study of belzutifan (MK6482), an oral hypoxia-inducible factor 2alpha (HIF-2alpha) inhibitor, for von Hippel-Lindau (VHL) disease associated clear cell renal cell carcinoma (ccRCC) [abstract no. 4555]. J Clin Oncol. 2021;39(15 Suppl):4555.
Wiley H, Coleman HR, Jonasch E, et al. Oral HIF-2α inhibitor belzutifan for ocular von Hippel-Lindau (VHL) disease. Investig Ophthalmol Visual Sci Conf. 2021;62(8):32.
Choueiri TK, Bauer TM, McDermott DF, et al. Phase 2 study of the oral hypoxiainducible factor 2alpha (HIF-2alpha) inhibitor MK-6482 in combination with cabozantinib in patients with advanced clear cell renal cell carcinoma (ccRCC) [abstract no. 272]. J Clin Oncol. 2021;39(6 Suppl):272.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Emma Deeks is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Belzutifan: First Approval. Drugs 81, 1921–1927 (2021). https://doi.org/10.1007/s40265-021-01606-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01606-x