Abstract
Background
Chronic hepatitis B is a common, progressive disease, particularly when viral replication is detected. Oral antivirals can suppress viral replication and prevent or delay the development of cirrhosis and liver-related complications.
Objective
The aim of this study was to systematically review the quality of cost-effectiveness evidence on first-line treatment with entecavir (ETV) or tenofovir difumarate (TDF) for patients with chronic hepatitis B.
Methods
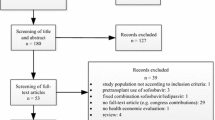
We searched electronic databases and retrieved articles published up to October 2011, in which the cost effectiveness of ETV or TDF was compared with that of other oral antivirals. The quality of the studies identified was assessed with a standard checklist for critical appraisal.
Results
We selected 16 original papers, all published in the last 5 years. There was a conflict of interest in 12 of the 16 studies due to sponsorship by the corresponding pharmaceutical companies. According to the validity assessment, ten studies were classified as high quality. Five studies performed a cost-effectiveness analysis comparing ETV with TDF; they concluded that TDF dominates ETV. The other 11 studies compared ETV or TDF with other strategies; all concluded that ETV and TDF are both cost-effective interventions.
Conclusions
This systematic review shows that there is valid evidence suggesting that ETV and TDF are cost-effective interventions for the treatment of patients with chronic hepatitis B in many health systems. In countries where both alternatives are available, it appears that TDF dominates ETV. These results could help decision makers and clinicians to understand economic issues regarding the available drugs for first-line treatment of hepatitis B.

Similar content being viewed by others
References
Ganem D, Prince AM. Hepatitis B virus infection: natural history and clinical consequences. N Engl J Med. 2004;350:1118–29.
Lok ASF, McMahon BJ. Chronic hepatitis B: AASLD practice guidelines. Hepatology. 2007;45:507–39.
Hoofnagle JH, Doo E, Liang TJ, et al. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–75.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–42.
Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73.
Mommeja-Marin H, Mondou E, Blum MR, et al. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology. 2003;37:1309–19.
Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;3:263–83.
Brown R, Cock E, Colin X, et al. Hepatitis B management costs in France, Italy, Spain and the United Kingdom. J Clin Gastroenterol. 2004;38:S169–74.
Idris BI, Brosa M, Richardus JH, et al. Estimating the future health burden of chronic hepatitis B and the impact of therapy in Spain. Eur J Gastroenterol Hepatol. 2008;20:320–6.
Simoens S. How to assess the value of medicines? Front Pharmacol. 2010;1:115.
García-Altés A, Navas E, Soriano MA. Economic evaluation of public health interventions [in Spanish]. Gac Sanit. 2011;25(Suppl. 1):25–31.
Ijzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access: a review of methods and applications. Appl Health Econ Health Policy. 2011;9(5):331–47.
Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: University of York; 2008. http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf. Accessed 29 Sept 2011.
Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. New York: Oxford University Press; 2005.
Arnold E, Yuan Y, Iloeje U, et al. Cost-effectiveness analysis of entecavir versus lamivudine in the first-line treatment of Australian patients with chronic hepatitis B. Appl Health Econ Health Policy. 2008;6(4):231–46.
Buti M, Brosa M, Casado MA, et al. Modeling the cost-effectiveness of different oral antiviral therapies in patients with chronic hepatitis B. J Hepatol. 2009;51(4):640–6.
Colombo GL, Gaeta GB, Vigano M, et al. A cost-effectiveness analysis of different therapies in patients with chronic hepatitis B in Italy. Clin Outcomes Res. 2011;3(1):37–46.
Costa AM, L ‘italien G, Nita ME, et al. Cost-effectiveness of entecavir versus lamivudine for the suppression of viral replication in chronic hepatitis B patients in Brazil. Braz J Infect Dis. 2008;12(5):368–73.
Dakin H, Bentley A, Dusheiko G. Cost-utility analysis of tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Value Health. 2010;13(8):922–33.
Dakin H, Sherman M, Fung S, et al. Cost effectiveness of tenofovir disoproxil fumarate for the treatment of chronic hepatitis B from a Canadian public payer perspective. Pharmacoeconomics. 2011;29(12):1075–91.
Kanwal F, Farid M, Martin P, et al. Treatment alternatives for hepatitis B cirrhosis: a cost-effectiveness analysis. Am J Gastroenterol. 2006;101(9):2076–89.
Lui YY, Tsoi KK, Wong VW, et al. Cost-effectiveness analysis of roadmap models in chronic hepatitis B using tenofovir as the rescue therapy. Antivir Ther. 2010;15(2):145–55.
Orlewska E, Zammit D, Yuan Y, et al. The cost-effectiveness analysis of entecavir in the treatment of chronic hepatitis B (CHB) patients in Poland. Exp Clin Hepatol. 2008;4(3–4):20–8.
Schwierz C, Thiry N, Van de Sande S, et al. Economic evaluation of antiviral treatment of chronic hepatitis B in Belgium: part 2 [KCE reports 157B]. Brussels: Belgian Health Care Knowledge Centre (KCE); 2011. http://test.kce.fgov.be/sites/default/files/page_documents/kce_157b_h%C3%A9patite%20b_chronique_tome%202.pdf. Accessed 10 Oct 2011.
Spackman DE, Veenstra DL. A cost-effectiveness analysis of currently approved treatments for HBeAg-positive chronic hepatitis B. Pharmacoeconomics. 2008;26(11):937–49.
Veenstra DL, Sullivan SD, Clarke L, et al. Cost effectiveness of entecavir versus lamivudine with adefovir salvage in HBeAg-positive chronic hepatitis B. Pharmacoeconomics. 2007;25(11):963–77.
Veenstra DL, Spackman DE, Di Bisceglie A, et al. Evaluating anti-viral drug selection and treatment duration in HBeAg-negative chronic hepatitis B: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2008;27(12):1240–52.
Wu B, Li T, Chen H, et al. Cost-effectiveness of nucleoside analog therapy for hepatitis B in China: a Markov analysis. Value Health. 2010;13(5):592–600.
Yuan Y, Iloeje U, Li H, et al. Economic implications of entecavir treatment in suppressing viral replication in chronic hepatitis B (CHB) patients in China from a perspective of the Chinese Social Security program. Value Health. 2008;11(Suppl 1):S11–22.
Yuan Y, Iloeje UH, Hay J, et al. Evaluation of the cost-effectiveness of entecavir versus lamivudine in hepatitis BeAg-positive chronic hepatitis B patients. J Manag Care Pharm. 2008;14(1):21–33.
Levy AR, Kowdley KV, Iloeje U, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health. 2008;11(3):527–38.
Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10.
Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–20.
Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73.
Iloeje UH, Yang SI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–86.
Dakin H, Fidler C, Harper C. Mixed treatment comparison meta-analysis evaluating the relative efficacy of nucleos(t)ides for treatment of nucleos(t)ide-naive patients with chronic hepatitis B. Value Health. 2010;13(8):934–45.
Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55.
Lai CL, Rosmawati M, Lao J, et al. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology. 2002;123:1831–8.
Thomas HC, Manns PM, Gish RG, et al. Decline in serum HBV DNA and ALT normalization is associated with histological response among nucleoside naïve patients in entecavir phase III trials. J Hepatol. 2005;42(Suppl 2):192–3.
Fattovich G, Giustina G, Schalm S, et al. Occurrence of hepatocellular carcinoma and decompensation in Western European patients with cirrhosis type B. Hepatology. 1995;21:77–82.
Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31.
Davis GL, Hoofnagle JH. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology. 1984;86:230–5.
Chan HLY, Hui AY, Wong ML, et al. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494–8.
Heathcote EJ, Gane EJ, de Man RA, et al. Two year tenofovir disoproxil fumarate (TDF) treatment and adefovir dipivoxil (ADV) switch data in HBeAg-negative patients with chronic hepatitis B (study 102), preliminary analysis [abstract no. 158]. In: 59th Annual Meeting of the American Association for the Study of Liver Diseases. 31 Oct–4 Nov 2008. San Francisco.
Marcellin P, Buti M, Krastev Z, et al. Two year tenofovir disoproxil fumarate (TDF) treatment and adefovir dipivoxil (ADV) switch data in HBeAg-negative patients with chronic hepatitis B (study 102) [abstract no. 146]. In: 59th Annual Meeting of the American Association for the Study of Liver Diseases. 31 Oct–4 Nov 2008. San Francisco.
Gish RG, Lok AS, Chang TT, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133(5):1437–44.
Yao G, Chen C, Lu W, et al. Efficacy and safety of entecavir compared to lamivudine in nucleoside-naïve patients with chronic hepatitis B: a randomized double-blind trial in China. Hepatol Int. 2007;1(3):365–7.
Shouval D, Lai CL, Chang TT, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50(2):289–95.
Horsmans Y, Thiry N, le Polain M, et al. Cost-effectiveness of antiviral treatment of chronic hepatitis B in Belgium. Part 1: literature review and results of a national study [KCE reports 127C]. Brussels: Belgian Health Care Knowledge Centre (KCE); 2010. https://kce.fgov.be/sites/default/files/page_documents/d20101027324.pdf. Accessed 10 Oct 2011.
Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–86.
Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10.
Gish R, Chang T, De Man RA, et al. Entecavir results in substantial virologic and biochemical improvement and HBeAg seroconversion through 96 weeks of treatment in HBeAg(+) chronic hepatitis B patients (study ETV-022). In: 56th American Association for the Study of Liver Diseases Annual Meeting; 11–15 Nov 2005; San Francisco.
Liaw YF, Tai DI, Chu CM, et al. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493–6.
Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–7.
Fattovich G, Pantalena M, Zagni I, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97:2886–95.
Lin YT, Liu YH. Epidemiological study of hepatitis B in 1990–2003 in Wuxi City, Jiangsu Province [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(12):1083.
Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–500.
Liaw YF, Nancy L, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–83.
Yao G. Entecavir is a potent anti-HBV drug superior to lamivudine: experience from clinical trials in China. J Antimicrob Chemother. 2007;60:201–5.
Sun X, Qin WX, Li YP, et al. Comparative cost-effectiveness of antiviral therapies in patients with chronic hepatitis B: a systematic review of economic evidence. J Gastroenterol Hepatol. 2007;22(9):1369–77.
Angus PW. Hepatitis B: weighing the costs of treatment. J Gastroenterol Hepatol. 2002;17(7):737–8.
Holbrook ML. Cost-effective pharmacologic therapies to treat chronic hepatitis B: how far have we really come? J Manag Care Pharm. 2008;14(1):65–9.
Anderson R. Systematic reviews of economic evaluations: utility or futility? Health Econ. 2010;19(3):350–64.
Carande-Kulis VG, Maciosek MV, Briss PA, et al. Methods for systematic reviews of economic evaluations for the Guide to Community Preventive Services. Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):75–91.
Jung KT, Shin DW, Lee KJ, et al. Cost-effectiveness of recombinant tissue plasminogen activator in the management of acute ischemic stroke: a systematic review. J Clin Neurol. 2010;6(3):117–26.
Mauskopf JA, Baker CL, Monz BU, et al. Cost effectiveness of tiotropium for chronic obstructive pulmonary disease: a systematic review of the evidence. J Med Econ. 2010;13(3):403–17.
Han SH. Natural course, therapeutic options and economic evaluation of therapies for chronic hepatitis B. Drugs. 2006;66(14):1831–51.
Briggs A. Transportability of comparative effectiveness and cost-effectiveness between countries. Value Health. 2010;13(Suppl. 1):S22–5.
Vemer P, Rutten-van Mölken MP. Largely ignored: the impact of the threshold value for a QALY on the importance of a transferability factor. Eur J Health Econ. 2011;12(5):397–404.
Acknowledgements
The authors thank Candido Gomez and Gema Mora for their collaboration and help in localizing the full-text papers.
Funding This study did not receive any funding. It was carried out independently by the authors.
Conflicts of Interest
MB has participated as a speaker and in advisory boards for Bristol-Myers Squibb (BMS), Gilead Sciences, MSD and Novartis. IO, VL and MAC are members of Pharmacoeconomics & Outcomes Research Iberia (PORIB), a consultancy company that usually works for pharmaceutical enterprises in economic evaluation projects.
Author Contributions
MB conceived the analysis and performed general coordination of the project. IO, MAC and MB designed and defined the search strategy. VL and IO performed the search, reviewed all the references, retrieved the selected publications, and performed a further review of them for data extraction. VL and IO drafted the manuscript. MB and MAC reviewed the draft manuscript and all authors agreed on the final version. MB, who had the idea for the paper, acts as guarantor for the overall content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buti, M., Oyagüez, I., Lozano, V. et al. Cost Effectiveness of First-Line Oral Antiviral Therapies for Chronic Hepatitis B . PharmacoEconomics 31, 63–75 (2013). https://doi.org/10.1007/s40273-012-0009-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-012-0009-2