Abstract
Objectives
The aim of this study was to develop an EQ-5D-5L value set reflecting the health preferences of the Malaysian adult population.
Methods
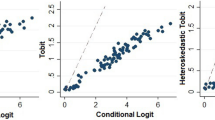
Respondents were sampled with quotas for urbanicity, gender, age, and ethnicity to ensure representativeness of the Malaysian population. The study was conducted using a standardized protocol involving the EuroQol Valuation Technology (EQ-VT) computer-assisted interview system. Respondents were administered ten composite time trade-off (C-TTO) tasks and seven discrete choice experiment (DCE) tasks. Both linear main effects and constrained non-linear regression models of C-TTO-only data and hybrid models combining C-TTO and DCE data were explored to determine an efficient and informative model for value set prediction.
Results
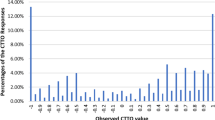
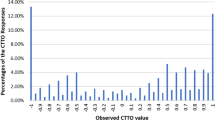
Data from 1125 respondents representative of the Malaysian population were included in the analysis. Logical consistency was present in all models tested. Using cross-validation, eight-parameter models for C-TTO only and C-TTO + DCE hybrid data displayed greater out-of-sample predictive accuracy than their 20-parameter, main-effect counterparts. The hybrid eight-parameter model was chosen to represent the Malaysian value set, as it displayed greater out-of-sample predictive accuracy over C-TTO data than the C-TTO-only model, and produced more precise estimates. The estimated value set ranged from − 0.442 to 1.
Conclusions
The constrained eight-parameter hybrid model demonstrated the best potential in representing the Malaysian value set. The presence of the Malaysian EQ-5D-5L value set will facilitate its application in research and health technology assessment activities.


Similar content being viewed by others
References
Devlin NJ, Brooks R. EQ-5D and the EuroQol Group: past, present and future. Appl Health Econ Health Policy. 2017;15(2):127–37. https://doi.org/10.1007/s40258-017-0310-5.
Wisløff T, Hagen G, Hamidi V, Movik E, Klemp M, Olsen JA. Estimating QALY gains in applied studies: a review of cost-utility analyses published in 2010. Pharmacoeconomics. 2014;32(4):367–75.
Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36. https://doi.org/10.1007/s11136-011-9903-x.
Brooks R, Rabin R, De Charro F. The measurement and valuation of health status using EQ-5D: a European perspective: evidence from the EuroQol BIO MED research programme. Berlin: Springer Science & Business Media; 2003.
Norman R, Cronin P, Viney R, King M, Street D, Ratcliffe J. International comparisons in valuing EQ-5D health states: a review and analysis. Value Health. 2009;12(8):1194–200. https://doi.org/10.1111/j.1524-4733.2009.00581.x.
Xie F, Gaebel K, Perampaladas K, Doble B, Pullenayegum E. Comparing EQ-5D valuation studies: a systematic review and methodological reporting checklist. Med Decis Mak. 2014;34(1):8–20. https://doi.org/10.1177/0272989x13480852.
Oppe M, Devlin NJ, van Hout B, Krabbe PFM, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health J Int Soc Pharmacoecon Outcomes Res. 2014;17(4):445–53.
Xie F, Pullenayegum E, Gaebel K, Bansback N, Bryan S, Ohinmaa A, et al. A time trade-off-derived value set of the EQ-5D-5L for Canada. Med Care. 2016;54(1):98–105. https://doi.org/10.1097/mlr.0000000000000447.
Augustovski F, Rey-Ares L, Irazola V, Garay O, Gianneo O, Fernández G, et al. An EQ-5D-5L value set based on Uruguayan population preferences. Qual Life Res. 2015;18(7):A810–1. https://doi.org/10.1016/j.jval.2015.09.104.
Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22. https://doi.org/10.1002/hec.3564.
Versteegh MM, Vermeulen KM, Evers SM, de Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19(4):343–52. https://doi.org/10.1016/j.jval.2016.01.003.
Kim S-H, Ahn J, Ock M, Shin S, Park J, Luo N, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res. 2016;25(7):1845–52. https://doi.org/10.1007/s11136-015-1205-2.
Ikeda S, Shiroiwa T, Igarashi A, Noto S, Fukuda T, Saito S. Developing a Japanese version of the EQ-5D-5L value set. J Natl Inst Public Health. 2015;64(1):47–55.
Ramos-Goñi JM, Pinto-Prades JL, Oppe M, Cabasés JM, Serrano-Aguilar P, Rivero-Arias O. Valuation and modeling of EQ-5D-5L health states using a hybrid approach. Med Care. 2014;55(7):e51–8. https://doi.org/10.1097/mlr.0000000000000283.
Luo N, Liu G, Li M, Guan H, Jin X, Rand-Hendriksen K. Estimating an EQ-5D-5L value set for China. Value Health. 2017;20(4):662–9. https://doi.org/10.1016/j.jval.2016.11.016.
Wong ELY, Ramos-Goñi JM, Cheung AWL, Wong AYK, Rivero-Arias O. Assessing the use of a feedback module to model EQ-5D-5L health states values in Hong Kong. Patient Patient Centered Outcomes Res. 2018;11(2):235–47. https://doi.org/10.1007/s40271-017-0278-0.
Purba FD, Hunfeld JAM, Iskandarsyah A, Fitriana TS, Sadarjoen SS, Ramos-Goñi JM, et al. The Indonesian EQ-5D-5L value set. Pharmacoeconomics. 2017;35(11):1153–65. https://doi.org/10.1007/s40273-017-0538-9.
Ludwig K, Graf von der Schulenburg J-M, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(6):663–74. https://doi.org/10.1007/s40273-018-0615-8.
Pattanaphesaj J, Thavorncharoensap M, Ramos-Goni JM, Tongsiri S, Ingsrisawang L, Teerawattananon Y. The EQ-5D-5L valuation study in Thailand. Expert Rev Pharmacoecon Outcomes Res. 2018;18(5):551–8. https://doi.org/10.1080/14737167.2018.1494574.
Hobbins A, Barry L, Kelleher D, Shah K, Devlin N, Goni JMR, et al. Utility values for health states in ireland: a value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(11):1345–53. https://doi.org/10.1007/s40273-018-0690-x.
Shafie AA. EuroQol 5-dimension measures in Malaysia. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. Springer: Berlin; 2014. p. 2041–4.
Oppe M, Van Hout B. The “power” of eliciting EQ-5D-5L values: the experimental design of the EQ-VT. EuroQol working paper series. 2017. p. 17003.
Department of Statistics Malaysia. Population distribution and basic demographic characteristic report 2010. https://www.statistics.gov.my/index.php?r=column/ctheme&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09&bul_id=MDMxdHZjWTk1SjFzTzNkRXYzcVZjdz09. Accessed 7 May 2015.
Ramos-Goñi JM, Oppe M, Slaap B, Busschbach JJV, Stolk E. Quality control process for EQ-5D-5L valuation studies. Value Health. 2017;20(3):466–73. https://doi.org/10.1016/j.jval.2016.10.012.
Oppe M, Rand-Hendriksen K, Shah K, Ramos-Goñi JM, Luo N. EuroQol protocols for time trade-off valuation of health outcomes. Pharmacoeconomics. 2016;34(10):993–1004. https://doi.org/10.1007/s40273-016-0404-1.
Bansback N, Brazier J, Tsuchiya A, Anis A. Using a discrete choice experiment to estimate health state utility values. J Health Econ. 2012;31(1):306–18. https://doi.org/10.1016/j.jhealeco.2011.11.004.
Stolk EA, Oppe M, Scalone L, Krabbe PF. Discrete choice modeling for the quantification of health states: the case of the EQ-5D. Value Health. 2010;13(8):1005–13.
Rand-Hendriksen K, Ramos-Goñi JM, Augestad LA, Luo N. Less is more: cross-validation testing of simplified nonlinear regression model specifications for EQ-5D-5L health state values. Value Health. 2017;20(7):945–52. https://doi.org/10.1016/j.jval.2017.03.013.
Oppe M, van Hout B, editors. The optimal hybrid: experimental design and modeling of a combination of TTO and DCE. In: 27th scientific plenary meeting of the EuroQol group-proceedings. Rotterdam: EuroQol Group Executive Office; 2010.
Shiroiwa T, Ikeda S, Noto S, Igarashi A, Fukuda T, Saito S, et al. Comparison of value set based on DCE and/or TTO data: scoring for EQ-5D-5L health states in Japan. Value Health. 2016;19(5):648–54. https://doi.org/10.1016/j.jval.2016.03.1834.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016.
Picard RR, Cook RD. Cross-validation of regression models. J Am Stat Assoc. 1984;79(387):575–83.
Arlot S, Celisse A. A survey of cross-validation procedures for model selection. Stat Surv. 2010;4:40–79.
Rowen D, Brazier J, Van Hout B. A comparison of methods for converting DCE values onto the full health-dead QALY scale. Med Decis Mak. 2015;35(3):328–40. https://doi.org/10.1177/0272989X14559542.
Acknowledgements
The authors thank the interviewers of the study for their dedication to the project. The authors would also like to express gratitude to Rhu Yann Ho, Elly Stolk, Kristina Ludwig, Jan J.V. Busschbach, Arnd Prause, and Juan Manuel Ramos Goñi, for their support during the study. The authors also thank Hospital Universiti Sains Malaysia, Hospital Sungai Buloh, and Segi College Subang Jaya for providing the facilities for this study. Lastly, we would like to thank the Director General of Health Malaysia for his permission to publish this article.
Funding
This study was co-funded by EuroQol Research Foundation and Universiti Sains Malaysia’s Research University Grant (1001/PFARMASI/811287).
Author information
Authors and Affiliations
Contributions
AAS designed the study and acquired the necessary funding. AAS, AVT, and CJL coordinated the data collection process, assisted by NL and FAMY. KR, NL, AAS, and AVT conceived the analysis. AAS, AVT, and CJL came up with the first draft of the manuscript. Every author contributed in reviewing the analysis, results, and recommended revisions to the final manuscript to ensure accuracy and fair balance.
Corresponding author
Ethics declarations
Conflict of interest
Nan Luo and Kim Rand-Hendriksen are members of the EuroQol Research Foundation. Asrul Akmal Shafie, Nan Luo, and Kim Rand-Hendriksen have received grants from EuroQol Research Foundation. There is no other conflict of interest.
Ethical approval
The study received ethical approval from the Malaysia Medical Research & Ethics Committee (ID NMRR-13-1377-18574) and was conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shafie, A.A., Vasan Thakumar, A., Lim, C.J. et al. EQ-5D-5L Valuation for the Malaysian Population. PharmacoEconomics 37, 715–725 (2019). https://doi.org/10.1007/s40273-018-0758-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0758-7