Abstract
Background
Atherosclerotic vascular disease represents a significant cause of morbidity and mortality in patients with end-stage renal disease (ESRD). The endothelium plays a crucial role in vascular inflammation. E-selectin is exclusively expressed on activated endothelial cells and is upregulated following an inflammatory response and oxidative stress, while serum pregnancy-associated plasma protein-A (PAPP-A) concentrations are related to the presence and stability of carotid atherosclerotic plaques.
Objective
The aim of this study was to investigate whether there is an association between SELE rs5355C>T gene polymorphism, serum PAPP-A level and the presence of carotid atherosclerosis in ESRD patients.
Subjects and Methods
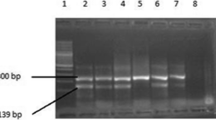
Seventy subjects were recruited into this study; 40 ESRD patients [age (mean ± SD) 43.42 ± 13.94 years] and 30 age- and gender-matched healthy individuals assigned to the control group. Polymerase chain reaction–restriction fragment length polymorphism was performed for the analysis of SELE rs5355C>T gene polymorphism, while serum PAPP-A concentrations were measured using electro-chemiluminescence immunoassay. Routine laboratory tests were measured on an automated chemistry analyzer. Carotid ultrasonographic studies were performed by a bilateral high-resolution B-mode ultrasound.
Results
There was no significant relationship between the SELE rs5355C>T gene polymorphism and ESRD incidence. Serum PAPP-A levels were significantly higher in ESRD patients compared with controls [median (interquartile range) 5.8 (5.1–11.6) and 5.1 (4.1–6.7), respectively; p = 0.005]. Serum PAPP-A correlated positively with urea, creatinine, systolic and diastolic blood pressure (DBP). Serum PAPP-A showed a statistically significant increase in SELE rs5355TT versus CC in both patients and controls. There was no association on comparing right intima-media thickness (IMT), left IMT, right cross-sectional area (CSA) and left CSA with the CC, CT and TT genotypes of SELE rs5355C>T. No correlation between serum PAPP-A with each of the above-mentioned carotid doppler findings was observed. There was a statistically significant increase in DBP in TT genotype carriers when compared with CC genotype carriers (p = 0.009). Serum PAPP-A levels were higher in hypertensive ESRD patients when compared with normotensive ESRD patients. There was a statistically significant decrease in high-density lipoprotein cholesterol (HDL-C) in TT genotype carriers when compared with CT genotype carriers in the whole study group (p = 0.003). Serum PAPP-A correlated negatively with HDL-C.
Conclusion
The lack of a direct association between SELE rs5355C>T gene polymorphism, serum PAPP-A level and IMT suggests that their hypothesized association with carotid atherosclerosis might reflect an indirect mechanism of SELE rs5355C>T gene polymorphism and serum PAPP-A with cardiovascular risk factors such as blood pressure and HDL-C rather than a direct effect on the vasculature.

Similar content being viewed by others
References
Foley R, Parfrey P, Sarnak M. Clinical epidemiology of cardiovascular disease in chronic renal failure. Am J Kidney Dis. 1998;32:S112–9.
Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2011;80(6):572–86. doi:10.1038/ki.2011.223.
Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26.
Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–46.
Roldan V, Marin F, Lip GY, et al. Soluble E-selectin in cardiovascular disease and its risk factors: a review of the literature. Thromb Haemost. 2003;90:1007–20.
Khazen D, Jendoubi-Ayed S, Aleya WB, et al. Polymorphism in ICAM-1, PECAM-1, E-selectin, and L-selectin genes in Tunisian patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21:167–75.
Malatino LS, Stancanelli1 B, Cataliotti A, et al. Circulating E-selectin as a risk marker in patients with end-stage renal disease. J Internal Med. 2007;262:479–87. doi:10.1111/j.1365-2796.2007.01841.x.
Ehret GB, O’Connor AA, Weder A, et al. Follow up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur J Hum Genet. 2009;17:1650–7.
Hunt SC, Ellison RC, Atwood LD, et al. Genome scans for blood pressure and hypertension: the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. 2002;40:1–6.
Marteau JB, Sass C, Pfister M, Lambert D, Noyer-Weidner M, Visvikis S. The Leu-554Phe polymorphism in the E-selectin gene is associated with blood pressure in overweight people. J Hypertens. 2004;22:305–11.
Wenzel K, Stahn R, Speer A, et al. Functional characterization of atherosclerosis-associated Ser-128Arg and Leu554Phe E-selectin mutations. Biol Chem. 1999;380:661–7.
Jilma B, Kovar FM, Hron G, et al. Homozygosity in the single nucleotide polymorphism Ser128Arg in the E-selectin gene associated with recurrent venous thromboembolism. Arch Intern Med. 2006;166(15):1655–9.
Ridker PM. Inflammation, atherosclerosis, and cardiovascular risk: an epidemiologic view. Blood Coagul Fibrinol. 1999;10 Suppl. 1:S9–12.
Conover CA, Harrington SC, Bale LK, et al. Surface association of pregnancy-associated plasma protein-A accounts for its colocalization with activated macrophages. Am J Physiol Heart Circ Physiol. 2007;292(2):H994–1000.
Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res. 2000;86:125–30.
Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14:176–81.
Tayebjee MH, Lip GY, MacFadyen RJ. Matrix metalloproteinases in coronary artery disease: clinical and therapeutic implications and pathological significance. Curr Med Chem. 2005;12:917–25.
Beaudeux JL, Burc L, Imbert-Bismut F, et al. Serum plasma pregnancy-associated protein A: a potential marker of echogenic carotid atherosclerotic plaques in asymptomatic hyperlipidemic subjects at high cardiovascular risk. Arteriosclerosis Thrombosis Vasc Biol. 2003;23:e7–10.
Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Horm IGF Res. 2008;18(3):213–20.
Miller SA, Dynes DD, Polesky F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res. 1988;16(3):1215.
Testa A, Benedetto FA, Spoto B, et al. The E-selectin gene polymorphism and carotid atherosclerosis in end-stage renal disease. Nephrol Dial Transpl. 2006;21:1921–6. doi:10.1093/ndt/gfl115.
Benedetto FA, Mallamaci F, Tripepi G, et al. Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol. 2001;12:2458–64.
Zoccali C, Mallamaci F, Tripepi G. Inflammation and atherosclerosis in end-stage renal disease. Blood Purif. 2003;21:29–36.
Hajilooi M, Alizadeh A-HM, Ranjbar M, et al. E-selectin gene polymorphisms in Iranian chronic hepatitis B patients. Hepat Mon. 2007;7(4):211–6.
Pallaud C, Gueguen R, Sass C, et al. Genetic influences on lipid metabolism trait variability within the Stanislas cohort. J Lipid Res. 2001;42:1879–91.
McLaren AJ, Marshall SE, Haldar NA, et al. Adhesion molecule polymorphisms in chronic renal allograft failure. Kidney Int. 1999;55:1977–82.
Weinstein J, de Souza-e-Silva U, Paulson JC. Purification of a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase and a Gal beta 1 to 3(4)GlcNAc alpha 2 to 3 sialyltransferase to homogeneity from rat liver. J Biol Chem. 1982;257:13835–44.
Revelle BM, Scott D, Beck PJ. Single amino acid residues in the E- and P-selectin epidermal growth factor domains can determine carbohydrate binding specificity. J Biol Chem. 1996;271:16160–70.
Wenzel K, Ernst M, Rohde K, et al. DNA polymorphisms in adhesion molecule genes: a new risk factor for early atherosclerosis. Hum Genet. 1996;97:15–20.
Haidari M, Hajilooi M, Rafiei AR, et al. E-selectin genetic variation as a susceptibility factor for ischemic stroke. Cerebrovasc Dis. 2009;28:26–32. doi:10.1159/000215940.
Kalousová M, Sulková S, Fialová L, et al. Glycoxidation and inflammation in chronic haemodialysis patients. Nephrol Dial Transplant. 2003;18:2577–2581. doi:10.1093/ndt/gfg404
Fialová l, Kalousová M, Soukupová J, et al. Relationship of pregnancy-associated plasma protein-a to renal function and dialysis modalities. Kidney Blood Pressure Res. 2004;27:88–95. doi:10.1159/000076390.
Coskun A, Bicik Z, Duran S, et al. Pregnancy-associated plasma protein A in dialysis patients. Clin Chem Lab Med. 2007;45(1):63–6.
Etter C, Straub Y, Hersberger M, et al. Pregnancy-associated plasma protein-A is an independent short-time predictor of mortality in patients on maintenance haemodialysis. Eur Heart J. 2010;31:354–9. doi:10.1093/eurheartj/ehp429.
Simon A, Gariepy J, Chironi G, et al. Intima media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20:159–69. doi:10.1097/00004872-200202000-00001.
Głowińska-Olszewska B, Tołwińska J, Urban M. Relationship between endothelial dysfunction, carotid artery intima media thickness and circulating markers of vascular inflammation in obese hypertensive children and adolescents. J Pediatr Endocrinol Metab. 2007;20(10):1125–36.
Rubio-Guerra AF, Vargas-Robles H, Serrano AM, et al. Correlation between the levels of circulating adhesion molecules and atherosclerosis in type-2 diabetic normotensive patients. Cell Adhesion Migr. 2009;3(4):369–72.
Woelfle J, Roth CL, Wunsch R, et al. Pregnancy-associated plasma protein A in obese children: relationship to markers and risk factors of atherosclerosis and members of the IGF system. Eur J Endocrinol. 2011;165:613–622.
Aso Y, Okumura K, Wakabayashi S, et al. Elevated pregnancy-associated plasma protein-A in sera from type 2 diabetic patients with hypercholesterolemia: associations with carotid atherosclerosis and toe-brachial index. J Clin Endocrinol Metab. 2004;89:5713–7.
Pellitero S, Reverter JL, Granada ML, et al. Association of the IGF1/pregnancy-associated plasma protein-A system and adipocytokine levels with the presence and the morphology of carotid plaques in type 2 diabetes mellitus patients with stable glycaemic control. Eur J Endocrinol. 2009;160:925–32.
Mueller T, Dieplinger B, Poelz W, et al. Increased pregnancy-associated plasma protein-A as a marker for peripheral atherosclerosis: results from the Linz Peripheral Arterial Disease Study. Clin Chem. 2006;52:1096–2103.
Faruque MU, Chen G, Doumatey A, Huang H, et al. Association of ATP1B1, RGS5 and SELE polymorphisms with hypertension and blood pressure in African–Americans. J Hypertens. 2011;29(10):1906–12.
Sass C, Pallaud C, Zannad F, et al. Association between E-selectin Leu554Phe polymorphism and blood pressure in the Stanislas cohort. Arch Mal Coeur Vaiss. 2001;94(8):855–8.
El Shamieh S, Ndiaye NC, Stathopoulou MG, Murray HA, Masson C, Lamont JV, Fitzgerald P, Benetos A, Visvikis-Siest S. Functional epistatic interaction between rs6046G>A in F7 and rs5355C>T in SELE modifies systolic blood pressure levels. PLoS One. 2012;7(7):e40777. doi:10.1371/journal.pone.0040777.
Cosin-Sales J, Kaski JC, Christiansen M, et al. Relationship among PAPP-A levels, clinical characteristics, and coronary artery disease extent in patients with chronic stable angina pectoris. Eur Heart J. 2005;26:2093–8.
Matsumoto K, Miyake S, Yano M, et al. High serum concentrations of soluble E-selectin in patients with impaired glucose tolerance with hyperinsulinemia. Atherosclerosis. 2000;152:415–20.
Barter PJ. Inhibition of endothelial cell adhesion molecule expression by high density lipoproteins. Clin Exp Pharmacol Physiol. 1997;24:286–7.
Calabresi L, Gomaraschi M, Villa B, et al. Elevated soluble cellular adhesion molecules in subjects with low HDL-cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:656–61. doi:10.1161/hq0402.105901.
Torres MR, Sanjuliani AF. Effects of weight loss from a high-calcium energy-reduced diet on biomarkers of inflammatory stress, fibrinolysis, and endothelial function in obese subjects. Nutrition. 2013;29(1):143–51. doi:10.1016/j.nut.2012.05.012.
Acknowledgements and Disclosures
The preparation of this manuscript was not supported by any external funding. The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Issac, M.S.M., Afif, A., Gohar, N.A. et al. Association of E-Selectin Gene Polymorphism and Serum PAPP-A with Carotid Atherosclerosis in End-Stage Renal Disease. Mol Diagn Ther 18, 243–252 (2014). https://doi.org/10.1007/s40291-013-0061-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-013-0061-4