Abstract
Purpose
In patients with TENIS syndrome (Thyroglobulin-Elevated Negative Iodine Scintigraphy), PET/CT radiotracers, such FDG PET/CT and somatostatin analogs (SSTR PET/CT), can identify disease localization. Therefore, the objective of this paper is to compare the capability of identifying lesions in TENIS Syndrome using FDG PET/CT and SSTR PET/CT through a systematic review and meta-analysis.
Methodology
A literature search was performed in electronic databases until February 2020 to identify relevant studies on FDG and 68 Ga-somatostatin analogs tracers in patients with TENIS Syndrome.
Results
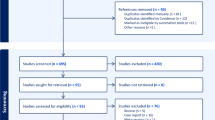
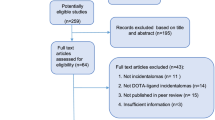
A total of 3551 abstracts reviewed from electronic databases and bibliographic references identified 13 candidates for the full-text analysis, and six articles were included in the qualitative and quantitative analyses. A total of 179 patients submitted to both SSTR PET/CT and FDG PET/CT to evaluate the performance of these radiotracers were analyzed. FDG PET/CT images identified significantly (p = 0.006) more patients with metastases when compared to SSTR PET/CT (143 vs. 103, respectively). Even though FDG PET/CT outperformed SSTR PET/CT, SSTR PET/CT was able to detect metastases in approximately 16% of FDG PET/CT negative cases.
Conclusion
FDG PET/CT has a higher capability to detect metastases than SSTR PET/CT in patients with TENIS Syndrome.







Similar content being viewed by others
Data availability
Not applicable.
References
Traub-Weidinger T, Putzer D, von Guggenberg E et al (2015) Multiparametric PET imaging in thyroid malignancy characterizing tumour heterogeneity: somatostatin receptors and glucose metabolism. Eur J Nucl Med Mol Imaging 42:1995–2001. https://doi.org/10.1007/s00259-015-3114-6
Pellegriti G, Frasca F, Regalbuto C et al (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013:1–10. https://doi.org/10.1155/2013/965212
Iñiguez-Ariza NM, Brito JP (2018) Management of low-risk papillary thyroid cancer. Endocrinol Metab 33:185. https://doi.org/10.3803/EnM.2018.33.2.185
Lan W, Gege Z, Ningning L et al (2019) Negative remnant 99mTc-pertechnetate uptake predicts excellent response to radioactive iodine therapy in low- to intermediate-risk differentiated thyroid cancer patients who have undergone total thyroidectomy. Ann Nucl Med 33:112–118. https://doi.org/10.1007/s12149-018-1314-4
Stokkel MPM, Duchateau CSJ, Dragoiescu C (2006) The value of FDG-PET in the follow-up of differentiated thyroid cancer: a review of the literature. Q J Nucl Med Mol Imaging 50:78–87
Avram AM, Rosculet N, Esfandiari NH et al (2019) Differentiated thyroid cancer outcomes after surgery and activity-adjusted 131I theragnostics. Clin Nucl Med 44:11–20. https://doi.org/10.1097/RLU.0000000000002321
Marcus C, Whitworth PW, Surasi DS et al (2014) PET/CT in the management of thyroid cancers. Am J Roentgenol 202:1316–1329. https://doi.org/10.2214/AJR.13.11673
Chung JK, So Y, Lee JS et al (1999) Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med 40:986–992
Wang W, Macapinlac H, Larson SM et al (1999) [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic131I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab 84:2291–2302. https://doi.org/10.1210/jcem.84.7.5827
Feine U, Lietzenmayer R, Hanke JP et al (1995) 18FDG whole-body PET in differentiated thyroid carcinoma. Flipflop in uptake patterns of 18FDG and 131I. Nuklearmedizin 34:127–134
Pisarek H, Stępień T, Kubiak R et al (2009) Expression of somatostatin receptor subtypes in human thyroid tumors: the immunohistochemical and molecular biology (RT-PCR) investigation. Thyroid Res 2:1. https://doi.org/10.1186/1756-6614-2-1
Klagge A, Krause K, Schierle K et al (2010) Somatostatin receptor subtype expression in human thyroid tumours. Horm Metab Res 42:237–240. https://doi.org/10.1055/s-0029-1243636
Kundu P, Lata S, Sharma P et al (2014) Prospective evaluation of 68Ga-DOTANOC PET-CT in differentiated thyroid cancer patients with raised thyroglobulin and negative 131I-whole body scan: Comparison with 18F-FDG PET-CT. Eur J Nucl Med Mol Imaging 41:1354–1362. https://doi.org/10.1007/s00259-014-2723-9
Shinto A, Samuel G, Velayutham P et al (2015) Utility of 99m Tc-Hynic-TOC in 131I whole-body scan negative thyroid cancer patients with elevated serum thyroglobulin levels. World J Nucl Med 14:101. https://doi.org/10.4103/1450-1147.154229
Rodrigues M, Li S, Gabriel M et al (2006) 99mTc-depreotide scintigraphy versus 18F-FDG-PET in the diagnosis of radioiodine-negative thyroid cancer. J Clin Endocrinol Metab 91:3997–4000. https://doi.org/10.1210/jc.2006-0825
Middendorp M, Selkinski I, Happel C et al (2010) Comparison of positron emission tomography with [(18)F]FDG and [(68)Ga]DOTATOC in recurrent differentiated thyroid cancer: preliminary data. Q J Nucl Med Mol Imaging 54:76–83
Le PS, Konopka T, Gacquer D et al (2015) Intratumor heterogeneity and clonal evolution in an aggressive papillary thyroid cancer and matched metastases. Endocr Relat Cancer 22:205–216. https://doi.org/10.1530/ERC-14-0351
Chmielik E, Rusinek D, Oczko-Wojciechowska M et al (2018) Heterogeneity of thyroid cancer. Pathobiology 85:117–129. https://doi.org/10.1159/000486422
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Pauwels E, Cleeren F, Bormans G, Deroose CM (2018) Somatostatin receptor PET ligands-the next generation for clinical practice. Am J Nucl Med Mol Imaging 8:311–331
National Institute of Health (2020) NIH Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 1 May 2020.
Rücker G, Schwarzer G, Carpenter J, Olkin I (2009) Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med 28:721–738. https://doi.org/10.1002/sim.3511
Wallace BC, Dahabreh IJ, Trikalinos TA et al (2012) Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 49:1–15. https://doi.org/10.18637/jss.v049.i05
Binse I, Poeppel TD, Ruhlmann M et al (2016) 68Ga-DOTATOC PET/CT in patients with iodine- and 18F-FDG-negative differentiated thyroid carcinoma and elevated serum thyroglobulin. J Nucl Med 57:1512–1517. https://doi.org/10.2967/jnumed.115.171942
Torun N, Erhmamci S, Reyhan M et al (2018) The clinic role of FDG-PET/CT and Ga68-DOTATATE PET/CT in differentiated thyroid cancer patients with negative iodine-131 whole body scan and elevated serum thyroglobulin level. Eur Assoc Nucl Med. https://doi.org/10.1007/s00259-018-4148-3
Versari A, Sollini M, Frasoldati A et al (2014) Differentiated thyroid cancer: A new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid 24:715–726. https://doi.org/10.1089/thy.2013.0225
Druckenthaner M, Schwarzer C, Ensinger C et al (2007) Evidence for Somatostatin receptor 2 in thyroid tissue. Regul Pept 138:32–39. https://doi.org/10.1016/j.regpep.2006.08.005
Wang Y, Wang W, Jin K et al (2017) Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol Lett 13:1165–1174. https://doi.org/10.3892/ol.2017.5591
Antunes P, Ginj M, Zhang H et al (2007) Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging 34:982–993. https://doi.org/10.1007/s00259-006-0317-x
Ganly I, Nixon IJ, Wang LY et al (2015) Survival from differentiated thyroid cancer: What has age got to do with it? Thyroid 25:1106–1114. https://doi.org/10.1089/thy.2015.0104
Kauffmann RM, Hamner JB, Ituarte PHG, Yim JH (2018) Age greater than 60 years portends a worse prognosis in patients with papillary thyroid cancer: Should there be three age categories for staging? BMC Cancer 18:1–10. https://doi.org/10.1186/s12885-018-4181-4
Nilubol N, Zhang L, Kebebew E (2013) Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid 23:695–702. https://doi.org/10.1089/thy.2012.0269
Katoh H, Yamashita K, Enomoto T, Watanabe M (2015) Annals of clinical pathology classification and general considerations of thyroid cancer. Ann Clin Pathol 3:1–9
Araz M, Çayır D (2017) 18F-Fluorodeoxyglucose-positron emission tomography/computed tomography for other thyroid cancers: medullary, anaplastic, lymphoma and so forth. Mol Imaging Radionucl Ther 26:1–8. https://doi.org/10.4274/mirt.60783
Carmona Matos DM, Jang S, Hijaz B et al (2019) Characterization of somatostatin receptors (SSTRs) expression and antiproliferative effect of somatostatin analogues in aggressive thyroid cancers. Surgery (United States) 165:64–68. https://doi.org/10.1016/j.surg.2018.05.077
Lamartina L, Borget I, Mirghani H et al (2017) Surgery for neck recurrence of differentiated thyroid cancer: Outcomes and risk factors. J Clin Endocrinol Metab 102:1020–1031. https://doi.org/10.1210/jc.2016-3284
Poeppel TD, Binse I, Petersenn S et al (2013) Differential Uptake of 68Ga-DOTATOC and 68Ga-DOTATATE in PET/CT of Gastroenteropancreatic Neuroendocrine Tumors. Springer, Berlin, pp 353–371. https://doi.org/10.1007/978-3-642-27994-2_18
Funding
None to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mourato, F.A., Almeida, M.A., Brito, A.E.T. et al. FDG PET/CT versus somatostatin receptor PET/CT in TENIS syndrome: a systematic review and meta-analysis. Clin Transl Imaging 8, 365–375 (2020). https://doi.org/10.1007/s40336-020-00390-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-020-00390-0