Abstract
Purpose
Prenatal androgen exposure could be a source of early programming, leading to the development of cardiometabolic diseases in later life. In this study, we aimed to examine cardiometabolic disturbances in males exposed to maternal androgen excess during their prenatal life.
Methods
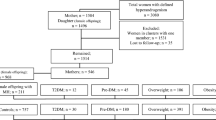
In this prospective population-based study, 409 male offspring with maternal hyperandrogenism (MHA), and 954 male offspring without MHA, as controls, were included. Both groups of male offspring were followed from the baseline to the date of the incidence of events, censoring, or end of the study period, whichever came first. Age-scaled unadjusted and adjusted Cox regression models were applied to assess the hazard ratios (HR) and 95% confidence intervals (CIs) for the association between MHA with pre-diabetes mellitus (Pre-DM), type 2 diabetes mellitus (T2DM), pre-hypertension (Pre-HTN), hypertension (HTN), dyslipidemia, overweight, and obesity in the offspring of both groups. Statistical analysis was performed using the STATA software package; the significance level was set at P < 0.05.
Results
A higher risk of Pre-DM (adjusted HR: 1.46 (1.20, 1.78)) was observed in male offspring with MHA after adjustment for potential confounders, including body mass index, education, and physical activity. However, no significant differences were observed in the risk of T2DM, Pre-HTN, HTN, dyslipidemia, overweight, and obesity in males with MHA compared to controls in both the unadjusted and adjusted models.
Conclusion
Maternal androgen excess increases the risk of Pre-DM in male offspring in later life. More longitudinal studies with long enough follow-up are needed to clarify the effects of MHA on the cardiometabolic risk factors of male offspring in later life.


Similar content being viewed by others
Data availability
Additional data may reasonably be requested from the corresponding author.
References
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, De Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD (2015) Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131:e29–e322. https://doi.org/10.1161/CIR.0000000000000152
Kwon EJ, Kim YJ (2017) What is fetal programming?: a lifetime health is under the control of in utero health. Obstet Gynecol Sci 60:506–519. https://doi.org/10.5468/ogs.2017.60.6.506
Recabarren SE, Petermann T, Maliqueo M, Lobos A, Rojas-García P (2006) Prenatal exposure to androgens as a factor of fetal programming. Rev Med Chile 134:101–108. https://doi.org/10.4067/S0034-98872006000100015
Hakim C, Padmanabhan V, Vyas AK (2017) Gestational hyperandrogenism in developmental programming. Endocrinology 158:199–212. https://doi.org/10.1210/en.2016-1801
Puttabyatappa M, Sargis RM, Padmanabhan V (2020) Developmental programming of insulin resistance: are androgens the culprits? J Endocrinol 245:R23–R48. https://doi.org/10.1530/JOE-20-0044
Carrasco A, Recabarren MP, Rojas-García PP, Silva N, Fuenzalida J, Sir-Petermann T, Recabarren SE (2021) Insulin sensitivity in male sheep born to ewes treated with testosterone during pregnancy. J Dev Orig Health Dis 12:456–464. https://doi.org/10.1017/S2040174420000665
Carrasco A, Recabarren MP, Rojas-García PP, Gutiérrez M, Morales K, Petermann T, Recabarren SE (2020) Prenatal testosterone exposure disrupts insulin secretion and promotes insulin resistance. Sci Rep 10:1–7. https://doi.org/10.1038/s41598-019-57197-x
Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburu B, Codner E, Cassorla F, RojasP Sir-Petermann T (2008) Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:1820–1826. https://doi.org/10.1210/jc.2007-2256
Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, Joe B, Hill JW (2018) Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 9:400–421. https://doi.org/10.1080/19490976.2018.1441664
Azizi F, Madjid M, Rahmani M, Emami H, Mirmiran P, Hadjipour R (2000) Tehran Lipid and Glucose Study (TLGS): rationale and design. Int J Endocrinol Metab 2:77–86
Tehrani FR, Behboudi-Gandevani S, Dovom MR, Farahmand M, Minooee S, Noroozzadeh M, Amiri M, Nazarpour S, Azizi F (2018) Reproductive assessment: findings from 20 years of the Tehran Lipid and Glucose Study. Int J Endocrinol Metab 16:84786. https://doi.org/10.5812/ijem.84786
Escobar-Morreale HF, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, Pugeat M, Qiao J, Wijeyaratne CN, Witchel SF, Norman RJ (2012) Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 18:146–70. https://doi.org/10.1093/humupd/dmr042
Tan AU, Schlosser BJ, Paller AS (2018) A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol. 4:56–71. https://doi.org/10.1016/j.ijwd.2017.10.006
Association EAD (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36:S67–S74. https://doi.org/10.2337/dc13-S067
Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek JE, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F (2008) Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes 32:959–966. https://doi.org/10.1038/ijo.2008.11
Chobanian AV (2003) National heart, lung, and blood institute; national high blood pressure education program coordinating committee. seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42:1206–1252. https://doi.org/10.1161/01.hyp.0000107251.49515.c2
Griffin BA, Anderson GL, Shih RA, Whitsel EA (2012) Use of alternative time scales in Cox proportional hazard models: implications for time-varying environmental exposures. Stat Med 31:3320–3327. https://doi.org/10.1002/sim.5347
Zhang Z (2016) Multiple imputation for time series data with Amelia package. Ann Transl Med 4:56. https://doi.org/10.3978/j.issn.2305-5839.2015.12.60
Skogen JC, Øverland S (2012) The fetal origins of adult disease: a narrative review of the epidemiological literature. JRSM 3:1–7. https://doi.org/10.1258/shorts.2012.012048
Barker DJP (2004) Developmental origins of well being. Philos Trans Royal Soc London 359:1359–66. https://doi.org/10.1098/rstb.2004.1518
Fowden AL, Forhead AJ (2004) Endocrine mechanisms of intrauterine programming. Reproduction 127:515–526. https://doi.org/10.1530/rep.1.00033
Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G (2006) Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol 126:16–19. https://doi.org/10.1016/j.ejogrb.2005.07.007
Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE (2002) Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 17:2573–2579. https://doi.org/10.1093/humrep/17.10.2573
Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH (1983) Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab 57:304–310. https://doi.org/10.1210/jcem-57-2-304
Guo S (2014) Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models to disease mechanisms. J Endocrinol 220:T1–T23. https://doi.org/10.1530/JOE-13-0327
Nilsson E, Ling C (2017) DNA methylation links genetics, fetal environment, and an unhealthy lifestyle to the development of type 2 diabetes. Clin Epigenetics 9:1–8. https://doi.org/10.1186/s13148-017-0399-2
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14:88–98. https://doi.org/10.1038/nrendo.2017.151
Franks PW, Pearson E, Florez JC (2013) Gene-environment and gene-treatment interactions in type 2 diabetes: progress, pitfalls, and prospects. Diabetes Care 36:1413–1421. https://doi.org/10.2337/dc12-2211
Vaiserman A, Lushchak O (2019) Developmental origins of type 2 diabetes: focus on epigenetics. Ageing Res Rev 55:100957. https://doi.org/10.1016/j.arr.2019.100957
Noroozzadeh M, Rahmati M, Behboudi-Gandevani S, Ramezani Tehrani F (2022) Maternal hyperandrogenism is associated with a higher risk of type 2 diabetes mellitus and overweight in adolescent and adult female offspring: a long-term population-based follow-up study. J Endocrinol Invest 45:963–972. https://doi.org/10.1007/s40618-021-01721-2
Tian S, Lin XH, Xiong YM, Liu ME, Yu TT, Lv M, Zhao W, Xu GF, Ding GL, Xu CM, Jin M (2017) Prevalence of prediabetes risk in offspring born to mothers with hyperandrogenism. EBioMedicine 16:275–83. https://doi.org/10.1016/j.ebiom.2017.01.011
Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburu B, Codner E, Cassorla F, Rojas P, Sir-Petermann T (2008) Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:1820–1826. https://doi.org/10.1210/jc.2007-2256
Chinnathambi V, Balakrishnan M, Yallampalli C, Sathishkumar K (2012) Prenatal testosterone exposure leads to hypertension that is gonadal hormone-dependent in adult rat male and female offspring. Biology of reproduction. Biol Reprod 86:137–7. https://doi.org/10.1095/biolreprod.103.023689
Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH (2004) Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab 89:6218–6223. https://doi.org/10.1210/jc.2004-0918
Lazic M, Aird F, Levine JE, Dunaif A (2011) Prenatal androgen treatment alters body composition and glucose homeostasis in male rats. J Endocrinol 208:293–300. https://doi.org/10.1677/JOE-10-0263
Huang G, Cherkerzian S, Loucks EB, Buka SL, Handa RJ, Lasley BL, Bhasin S, Goldstein JM (2018) Sex differences in the prenatal programming of adult metabolic syndrome by maternal androgens. J Clin Endocrinol Metab 103:3945–3953. https://doi.org/10.1210/jc.2014-2700
Zhang B, Wang J, Shen S, Liu J, Sun J, Gu T, Ye X, Zhu D, Bi Y (2018) Association of androgen excess with glucose intolerance in women with polycystic ovary syndrome. BioMed Res Int. https://doi.org/10.1155/2018/6869705
Osibogun O, Ogunmoroti O, Michos ED (2020) Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med 30:399–404. https://doi.org/10.1016/j.tcm.2019.08.010
Ramezani Tehrani F, Amiri M, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E (2020) Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol 36:12–23. https://doi.org/10.1080/09513590.2019.1650337
Forslund M, Schmidt J, Brännström M, Landin-Wilhelmsen K, Dahlgren E (2022) Morbidity and mortality in PCOS: A prospective follow-up up to a mean age above 80 years. Eur J Obstet Gynecol Reprod Biol 271:195–203. https://doi.org/10.1016/j.ejogrb.2022.02.020
Ashraf S, Nabi M, Rashid F, Amin S (2019) Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. EJMHG 20:1. https://doi.org/10.1186/s43042-019-0031-4
Franks S, McCarthy MI, Hardy K (2006) Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl 29:278–85. https://doi.org/10.1111/j.1365-2605.2005.00623.x
Barsky M, Merkison J, Hosseinzadeh P, Yang L, Bruno-Gaston J, Dunn J, Gibbons W, Blesson CS (2021) Fetal programming of polycystic ovary syndrome: Effects of androgen exposure on prenatal ovarian development. J Steroid Bioch Mol Biol 207:105830. https://doi.org/10.1016/j.jsbmb.2021.105830
Cesta CE, Öberg AS, Ibrahimson A, Yusuf I, Larsson H, Almqvist C, D’Onofrio BM, Bulik CM, de la Cruz LF, Mataix-Cols D, Landén M (2020) Maternal polycystic ovary syndrome and risk of neuropsychiatric disorders in offspring: prenatal androgen exposure or genetic confounding? Psychol Med 50:616–24. https://doi.org/10.1017/S0033291719000424
Daan NM, Koster MP, Steegers-Theunissen RP, Eijkemans MJ, Fauser BC (2017) Endocrine and cardiometabolic cord blood characteristics of offspring born to mothers with and without polycystic ovary syndrome. Fertil Steril 107:261–8. https://doi.org/10.1016/j.fertnstert.2016.09.042
Mehrabian F, Khani B (2014) Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. Int J Reprod BioMed 12:5
Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M, Hardiman PJ (2010) Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol 30:444–6. https://doi.org/10.3109/01443615.2010.485254
Palomba S, Russo T, Falbo A, Di Cello A, Tolino A, Tucci L, La Sala GB, Zullo F (2013) Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod 28:2838–47. https://doi.org/10.1093/humrep/det250
Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T (2013) Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 166:151–5. https://doi.org/10.1016/j.ejogrb.2012.10.015
Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V (2014) Developmental programing: impact of testosterone on placental differentiation. Reprod 148:199–209. https://doi.org/10.1530/REP-14-0055
Padmanabhan V, Veiga-Lopez A (2013) Animal models of the polycystic ovary syndrome phenotype. Steroids 78:734–40. https://doi.org/10.1016/j.steroids.2013.05.004
Funding
This work was financially supported by the project (No. 19868) of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
M.F.A. contributed substantially to the conception and design, interpretation of data, drafted the article and revised and approved the final version to be published. M.N. contributed substantially to the conception and design, interpretation of data, drafted the article and revised and approved the final version to be published. M.G. contributed substantially to interpretation of data, drafted the article and revised and approved the final version to be published. M.R. contributed substantially to analysis data, drafted the article, and revised and approved the final version to be published. M.S.G.N. contributed substantially to the draft of the article and revised and approved the final version to be published. F.A. contributed substantially to conception and design, revised and approved the final version to be published. F.R.T contributed substantially to conception and design, interpretation of data, drafting the article, and revising and approving the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
The ethics review board of the Research Institute for Endocrine Sciences approved the study proposal (approval number: IR.SBMU.ENDOCRINE.REC.1398.125).
Consent to participate
Written informed consent was signed by all participants after a full explanation of the purpose of the study to them. Written consent was obtained from their parents if they were under 18 years old.
Consent for publication
All authors approved the final version of the article for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farhadi-Azar, M., Noroozzadeh, M., Ghahremani, M. et al. Maternal androgen excess increases the risk of pre-diabetes mellitus in male offspring in later life: a long-term population-based follow-up study. J Endocrinol Invest 46, 1775–1785 (2023). https://doi.org/10.1007/s40618-022-01972-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01972-7